Page 66 - Adsorption, Ion Exchange & Catalysis- 2007, Elsevier - Copy
P. 66
Else_AIEC-INGLE_cH003.qxd 7/13/2006 1:44 PM Page 62
62 3. Heterogeneous Processes and Reactor Analysis
The rate coefficient is almost always dependent on temperature. However, it can be influ-
enced by total pressure, in both gas and liquid systems, plus ionic strength and solvent in liq-
uid systems. Following Fogler (1999), in the present book, the rate coefficient is considered
to be a function of only temperature, assuming that the effect of other variables is much less.
ement ang Reactor level—Catalyst plus r eactor arr
The principal difference between homogeneous and heterogeneous reaction rates is that the
, latter is based on mass, v or more rarely on the area of the solid and not on the fluid- olume,
phase volume or reactor volume. The reactor volume or liquid-phase volume is of second-
ary significance in heterogeneous reactions since the reaction takes place on the solid rather
than throughout the reactor volume. Moreover, the mass of the solid is usually used instead
. of the solid volume or surface, because it is the most easily measured property
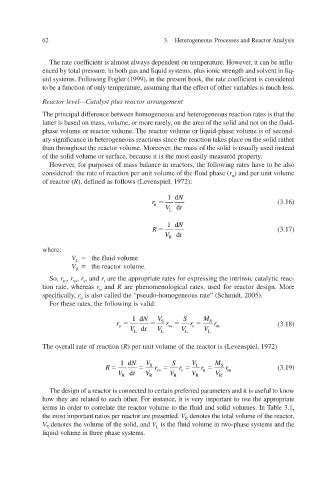
However, for purposes of mass balance in reactors, the following rates hae to be also v
considered: the rate of reaction per unit v olume of the fluid phase ( r ) and per unit volume u
of reactor ( R ), defined as follo 1972): v ws (Le enspiel,
1d N
r (3.16)
u
V L t d
1d N
R (3.17)
V R t d
where:
V L the fluid v olume
V R the reactor v olume.
So, r , r , r , and r are the appropriate rates for expressing the intrinsic catalytic reac-
vs
t
s
m
tion rate, whereas r u and R are phenomenological rates, used for reactor design. More
specifically, r is also called the “pseudo-homogeneous rate” (Schmidt, 2005).
u
For these rates, the following is v alid:
1d N V S M
r S r vs r S r m (3.18)
s
u
V L t d V L V L V L
v The oerall rate of reaction ( R ) per unit volume of the reactor is (Le 1972) v enspiel,
1d N V S V M
R S r vs r L r S r m (3.19)
s
u
V R t d V R V R V R V R
The design of a reactor is connected to certain preferred parameters and it is useful to know
how they are related to each other. For instance, it is v ery important to use the appropriate
terms in order to correlate the reactor volume to the fluid and solid v olumes. In able 3.1, T
the most important ratios per reactor are presented. V R denotes the total volume of the reactor,
V S denotes the volume of the solid, and V L is the fluid volume in two-phase systems and the
liquid-volume in three phase systems.