Page 1039 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 1039
carefully controlled conditions. Furthermore, fluorine atoms are capable of cleaving 1023
carbon-carbon bonds.
SECTION 11.3
F . + CH CH 3 CH F + CH 3 . Free Radical
3
3
Substitution Reactions
Saturated hydrocarbons such as neopentane, norbornane, and cyclooctane have been
converted to the corresponding perfluoro derivatives in 10–20% yield by reaction
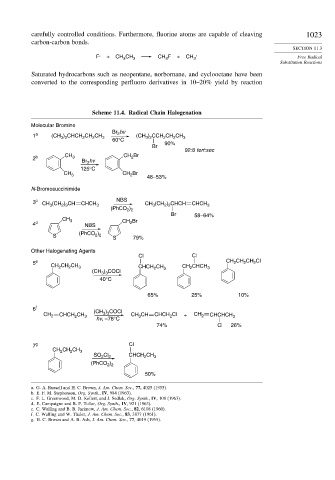
Scheme 11.4. Radical Chain Halogenation
Molecular Bromine
Br hv
1 a (CH ) CHCH CH CH 3 2 (CH ) CCH CH CH 3
2
2
3 2
2
2
3 2
60°C
Br 90%
92:8 tert:sec
2
2 b CH 3 CH Br
Br hv
2
125°C
CH 3 CH Br 48–53%
2
N-Bromosuccinimide
3 c CH (CH ) CH CHCH 3 NBS CH 3 (CH ) CHCH CHCH 3
3
2 2
2 3
(PhCO )
2 2
Br 58–64%
CH 3 CH Br
4 d NBS 2
)
S (PhCO 2 2
S 79%
Other Halogenating Agents
Cl Cl
5 e CH CH CH Cl
2
2
2
CH CH CH 3 CHCH CH 3 CH CHCH 3
2
2
2
2
(CH ) COCl
3 3
40°C
65% 25% 10%
6 f (CH ) COCl
CH 2 CHCH CH 3 3 3 CH CH CHCH Cl + CH 2 CHCHCH 3
2
3
2
hv, –78°C
74% Cl 26%
7 g Cl
CH CH CH 3
2
2
SO Cl 2 CHCH CH 3
2
2
(PhCO )
2 2
50%
a. G. A. Russell and H. C. Brown, J. Am. Chem. Soc., 77, 4025 (1955).
b. E. F. M. Stephenson, Org. Synth., IV, 984 (1963).
c. F. L. Greenwood, M. D. Kellert, and J. Sedlak, Org. Synth., IV, 108 (1963).
d. E. Campaigne and B. F. Tullar, Org. Synth., IV, 921 (1963).
e. C. Walling and B. B. Jacknow, J. Am. Chem. Soc., 82, 6108 (1960).
f. C. Walling and W. Thaler, J. Am. Chem. Soc., 83, 3877 (1961).
g. H. C. Brown and A. B. Ash, J. Am. Chem. Soc., 77, 4019 (1955).