Page 1044 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 1044
1028 .
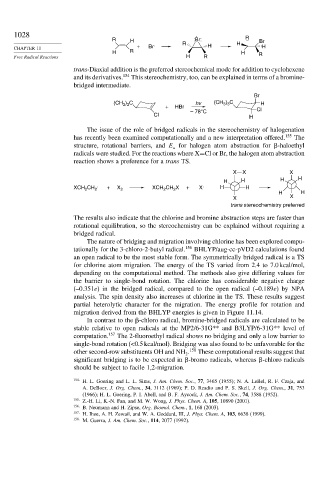
R H :Br: R Br
+ Br . R H H H
CHAPTER 11
H R H R
Free Radical Reactions H R
trans-Diaxial addition is the preferred stereochemical mode for addition to cyclohexene
and its derivatives. 154 This stereochemistry, too, can be explained in terms of a bromine-
bridged intermediate.
Br
(CH ) C hv (CH ) C H
3 3
3 3
+ HBr
– 78°C Cl
Cl H
The issue of the role of bridged radicals in the stereochemistry of halogenation
has recently been examined computationally and a new interpretation offered. 155 The
structure, rotational barriers, and E for halogen atom abstraction for -haloethyl
a
radicals were studied. For the reactions where X=Cl or Br, the halogen atom abstraction
reaction shows a preference for a trans TS.
X X X
H H H H
XCH CH 2 . + X 2 XCH CH X + X . H H
2
2
2
H H
X X
trans stereochemistry preferred
The results also indicate that the chlorine and bromine abstraction steps are faster than
rotational equilibration, so the stereochemistry can be explained without requiring a
bridged radical.
The nature of bridging and migration involving chlorine has been explored compu-
tationally for the 3-chloro-2-butyl radical. 156 BHLYP/aug-cc-pVD2 calculations found
an open radical to be the most stable form. The symmetrically bridged radical is a TS
for chlorine atom migration. The energy of the TS varied from 2.4 to 7.0 kcal/mol,
depending on the computational method. The methods also give differing values for
the barrier to single-bond rotation. The chlorine has considerable negative charge
(–0.351e) in the bridged radical, compared to the open radical (–0.189e)byNPA
analysis. The spin density also increases at chlorine in the TS. These results suggest
partial heterolytic character for the migration. The energy profile for rotation and
migration derived from the BHLYP energies is given in Figure 11.14.
In contrast to the -chloro radical, bromine-bridged radicals are calculated to be
stable relative to open radicals at the MP2/6-31G** and B3LYP/6-31G** level of
computation. 157 The 2-fluoroethyl radical shows no bridging and only a low barrier to
single-bond rotation (<0.5 kcal/mol). Bridging was also found to be unfavorable for the
other second-row substituents OH and NH . 158 These computational results suggest that
2
significant bridging is to be expected in -bromo radicals, whereas -chloro radicals
should be subject to facile 1,2-migration.
154
H. L. Goering and L. L. Sims, J. Am. Chem. Soc., 77, 3465 (1955); N. A. LeBel, R. F. Czaja, and
A. DeBoer, J. Org. Chem., 34, 3112 (1969); P. D. Readio and P. S. Skell, J. Org. Chem., 31, 753
(1966); H. L. Goering, P. I. Abell, and B. F. Aycock, J. Am. Chem. Soc., 74, 3588 (1952).
155
Z.-H. Li, K.-N. Fan, and M. W. Wong, J. Phys. Chem. A, 105, 10890 (2001).
156 B. Neumann and H. Zipse, Org. Biomol. Chem., 1, 168 (2003).
157 H. Ihee, A. H. Zewail, and W. A. Goddard, III, J. Phys. Chem. A, 103, 6638 (1999).
158
M. Guerra, J. Am. Chem. Soc., 114, 2077 (1992).