Page 1047 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 1047
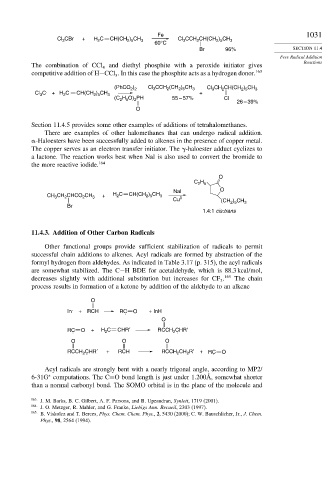
Fe 1031
Cl 3 CBr + H 2 C CH(CH 2 ) 5 CH 3 Cl CCH 2 CH(CH 2 ) 5 CH 3
3
60°C
Br 96% SECTION 11.4
Free Radical Addition
Reactions
The combination of CCl and diethyl phosphite with a peroxide initiator gives
4
competitive addition of H−CCl . In this case the phosphite acts as a hydrogen donor. 163
3
(PhCO ) Cl CCH (CH ) CH 3 Cl CH CH(CH ) CH 3
2 2
2 6
3
2
3
2
2 5
Cl C . + H C CH(CH ) CH 3 +
2
2 5
3
(C H O) PH 55 – 57% Cl
2
2 5
26 – 39%
O
Section 11.4.5 provides some other examples of additions of tetrahalomethanes.
There are examples of other halomethanes that can undergo radical addition.
-Haloesters have been successfully added to alkenes in the presence of copper metal.
The copper serves as an electron transfer initiator. The -haloester adduct cyclizes to
a lactone. The reaction works best when NaI is also used to convert the bromide to
the more reactive iodide. 164
O
C H
2 5
NaI O
CH CH CHCO CH 3 + H C CH(CH ) CH 3 0
2 5
2
2
2
3
Cu ) CH
(CH 2 5 3
Br
1.4:1 cis:trans
11.4.3. Addition of Other Carbon Radicals
Other functional groups provide sufficient stabilization of radicals to permit
successful chain additions to alkenes. Acyl radicals are formed by abstraction of the
formyl hydrogen from aldehydes. As indicated in Table 3.17 (p. 315), the acyl radicals
are somewhat stabilized. The C−H BDE for acetaldehyde, which is 88.3 kcal/mol,
decreases slightly with additional substitution but increases for CF . 165 The chain
3
process results in formation of a ketone by addition of the aldehyde to an alkene
O
.
In + RCH RC . O + InH
O
RC . O + H C CHR′ RCCH 2 CHR′
.
2
O O O
RCCH CHR′ + RCH RCCH 2 CH R′ + RC . O
2 .
2
Acyl radicals are strongly bent with a nearly trigonal angle, according to MP2/
∗
6-31G computations. The C=O bond length is just under 1.200Å, somewhat shorter
than a normal carbonyl bond. The SOMO orbital is in the plane of the molecule and
163 J. M. Barks, B. C. Gilbert, A. F. Parsons, and B. Upeandran, Synlett, 1719 (2001).
164 J. O. Metzger, R. Mahler, and G. Franke, Liebigs Ann. Recueil, 2303 (1997).
165
B. Viskolcz and T. Berces, Phys. Chem. Chem. Phys., 2, 5430 (2000); C. W. Bauschlicher, Jr., J. Chem.
Phys., 98, 2564 (1994).