Page 1052 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 1052
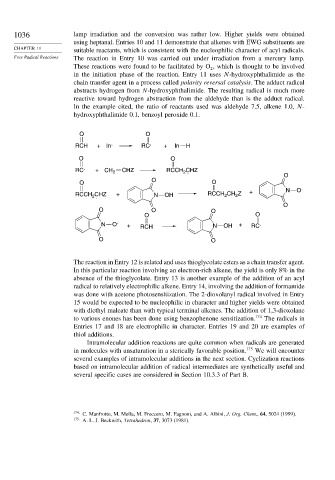
1036 lamp irradiation and the conversion was rather low. Higher yields were obtained
using heptanal. Entries 10 and 11 demonstrate that alkenes with EWG substituents are
CHAPTER 11 suitable reactants, which is consistent with the nucleophilic character of acyl radicals.
Free Radical Reactions The reaction in Entry 10 was carried out under irradiation from a mercury lamp.
These reactions were found to be facilitated by O , which is thought to be involved
2
in the initiation phase of the reaction. Entry 11 uses N-hydroxyphthalimide as the
chain transfer agent in a process called polarity reversal catalysis. The adduct radical
abstracts hydrogen from N-hydroxyphthalimide. The resulting radical is much more
reactive toward hydrogen abstraction from the aldehyde than is the adduct radical.
In the example cited, the ratio of reactants used was aldehyde 7.5, alkene 1.0, N-
hydroxyphthalimide 0.1, benzoyl peroxide 0.1.
O O
RCH +In . RC . + In H
O O
RC . + CH 2 CHZ RCCH CHZ
2 .
O
O
O O .
RCCH CHZ + N OH RCCH CH Z + N O
2
2
2 .
O
O O O
O O
N O . + RCH N OH + RC .
O O
The reaction in Entry 12 is related and uses thioglycolate esters as a chain transfer agent.
In this particular reaction involving an electron-rich alkene, the yield is only 8% in the
absence of the thioglycolate. Entry 13 is another example of the addition of an acyl
radical to relatively electrophilic alkene. Entry 14, involving the addition of formamide
was done with acetone photosensitization. The 2-dioxolanyl radical involved in Entry
15 would be expected to be nucleophilic in character and higher yields were obtained
with diethyl maleate than with typical terminal alkenes. The addition of 1,3-dioxolane
to various enones has been done using benzophenone sensitization. 174 The radicals in
Entries 17 and 18 are electrophilic in character. Entries 19 and 20 are examples of
thiol additions.
Intramolecular addition reactions are quite common when radicals are generated
in molecules with unsaturation in a sterically favorable position. 175 We will encounter
several examples of intramolecular additions in the next section. Cyclization reactions
based on intramolecular addition of radical intermediates are synthetically useful and
several specific cases are considered in Section 10.3.3 of Part B.
174 C. Manfrotto, M. Mella, M. Freccero, M. Fagnoni, and A. Albini, J. Org. Chem., 64, 5024 (1999).
175
A. L. J. Beckwith, Tetrahedron, 37, 3073 (1981).