Page 1055 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 1055
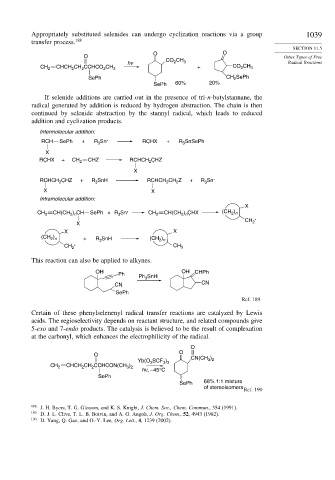
Appropriately substituted selenides can undergo cyclization reactions via a group 1039
transfer process. 188
SECTION 11.5
O O
O Other Types of Free
2
hv CO CH 3 Radical Reactions
CH 2 CHCH CH CCHCO CH 3 + CO 2 CH 3
2
2
2
SePh CH SePh
2
SePh 60% 20%
If selenide additions are carried out in the presence of tri-n-butylstannane, the
radical generated by addition is reduced by hydrogen abstraction. The chain is then
continued by selenide abstraction by the stannyl radical, which leads to reduced
addition and cyclization products.
Intermolecular addition:
RCH SePh + R Sn . RCHX + R SnSePh
.
3
3
X
RCHX + CH 2 CHZ RCHCH CHZ
2 .
.
X
RCHCH CHZ + R SnH RCHCH CH Z + R Sn .
2 .
2
2
3
3
X X
Intramolecular addition:
X
.
CH 2 CH(CH ) CH SePh +R Sn CH 2 CH(CH ) CHX (CH )
2 n
2 n .
3
2 n
CH .
X 2
X X
(CH ) + R SnH (CH 2 n
)
2 n
3
.
CH 2 CH 3
This reaction can also be applied to alkynes.
OH OH CHPh
Ph Ph SnH
3
CN CN
SePh
Ref. 189
Certain of these phenylselenenyl radical transfer reactions are catalyzed by Lewis
acids. The regioselectivity depends on reactant structure, and related compounds give
5-exo and 7-endo products. The catalysis is believed to be the result of complexation
at the carbonyl, which enhances the electrophilicity of the radical.
O
O
O
CN(CH )
3 2
Yb(O 3 SCF 3 ) 3
2
2
CH 2 CHCH CH CCHCON(CH )
3 2
hv, –45°C
SePh
SePh 68% 1:1 mixture
of stereoisomers Ref. 190
188
J. H. Byers, T. G. Gleason, and K. S. Knight, J. Chem. Soc., Chem. Commun., 354 (1991).
189 D. J. L. Clive, T. L. B. Boivin, and A. G. Angoh, J. Org. Chem., 52, 4943 (1982).
190
D. Yang, Q. Gao, and O.-Y. Lee, Org. Lett., 4, 1239 (2002).