Page 1058 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 1058
1042 bridged radicals formed by an addition process. In the case of aryl migration, the
bridged intermediate is a cyclohexadienyl radical.
CHAPTER 11
Free Radical Reactions .
. C
C C . C C C
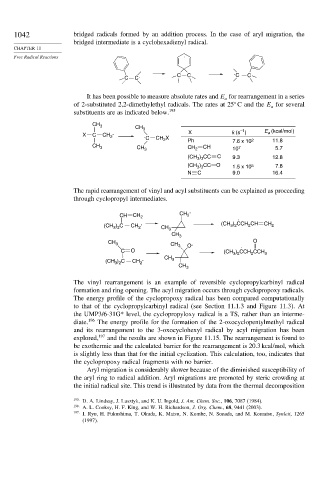
It has been possible to measure absolute rates and E for rearrangement in a series
a
of 2-substituted 2,2-dimethylethyl radicals. The rates at 25 C and the E for several
a
substituents are as indicated below. 195
CH 3
CH 3 –1 E (kcal/mol)
X
X C CH 2 . . C CH X Ph k (s ) 2 a 11.8
2
7.6 x 10
CH 3 CH 2 10 7 5.7
CH 3 CH
(CH ) CC C 9.3 12.8
3 3
(CH ) CC O 1.5 x 10 5 7.8
3 3
N C 9.0 16.4
The rapid rearrangement of vinyl and acyl substituents can be explained as proceeding
through cyclopropyl intermediates.
.
CH CH 2 CH 2 .
) CCH CH
(CH ) C CH 2 . CH 3 (CH 3 2 2 CH 2
3 2
CH 3
CH 3 CH 3 O . . O
C O (CH ) CCH CCH 3
2
3 2
. CH 3
(CH ) C CH 2
3 2
CH 3
The vinyl rearrangement is an example of reversible cyclopropylcarbinyl radical
formation and ring opening. The acyl migration occurs through cyclopropoxy radicals.
The energy profile of the cyclopropoxy radical has been compared computationally
to that of the cyclopropylcarbinyl radical (see Section 11.1.3 and Figure 11.3). At
the UMP3/6-31G* level, the cyclopropyloxy radical is a TS, rather than an interme-
diate. 196 The energy profile for the formation of the 2-oxocyclopentylmethyl radical
and its rearrangement to the 3-oxocyclohexyl radical by acyl migration has been
explored, 197 and the results are shown in Figure 11.15. The rearrangement is found to
be exothermic and the calculated barrier for the rearrangement is 20.3 kcal/mol, which
is slightly less than that for the initial cyclization. This calculation, too, indicates that
the cyclopropoxy radical fragments with no barrier.
Aryl migration is considerably slower because of the diminished susceptibility of
the aryl ring to radical addition. Aryl migrations are promoted by steric crowding at
the initial radical site. This trend is illustrated by data from the thermal decomposition
195 D. A. Lindsay, J. Lusztyk, and K. U. Ingold, J. Am. Chem. Soc., 106, 7087 (1984).
196 A. L. Cooksy, H. F. King, and W. H. Richardson, J. Org. Chem., 68, 9441 (2003).
197
I. Ryu, H. Fukushima, T. Okuda, K. Matsu, N. Kombe, N. Sonada, and M. Komatsu, Synlett, 1265
(1997).