Page 1060 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 1060
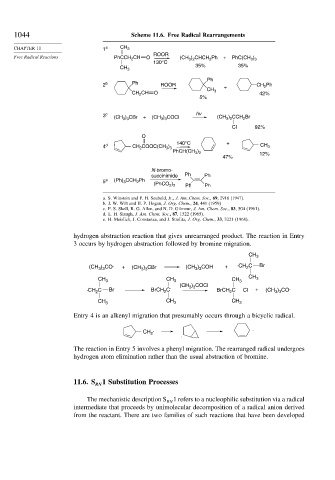
1044 Scheme 11.6. Free Radical Rearrangements
CHAPTER 11 1 a CH 3
ROOR
Free Radical Reactions PhCCH 2 CH O (CH 3 ) 2 CHCH 2 Ph + PhC(CH 3 ) 3
130°C
35% 35%
CH 3
Ph
2 b Ph ROOR + CH 2 Ph
CH 3
CH 2 CH O 42%
5%
3 c (CH 3 ) 3 CBr + (CH 3 ) 3 COCl hv (CH 3 ) 2 CCH 2 Br
Cl 92%
O
140°C +
4 d CH 2 COOC(CH 3 ) 3 CH 3
PhCH(CH 3 ) 2 12%
47%
N-bromo-
succinimide Ph Ph
5 e (Ph) 3 CCH 2 Ph
(PhCO 2 ) 2 Ph Ph
a. S. Winstein and F. H. Seubold, Jr., J. Am..Chem. Soc., 69, 2916 (1947).
b. J. W. Wilt and H. P. Hogan, J. Org. Chem., 24, 441 (1959)
c. P. S. Skell, R. G. Allen, and N. D. Gilmour, J. Am. Chem. Soc., 83, 504 (1961).
d. L. H. Slaugh, J. Am. Chem. Soc.,87, 1522 (1965).
e. H. Meislich, J. Constanza, and J. Strelitz, J. Org. Chem., 33, 3221 (1968).
hydrogen abstraction reaction that gives unrearranged product. The reaction in Entry
3 occurs by hydrogen abstraction followed by bromine migration.
CH 3
) COH
(CH ) CO . + (CH ) CBr (CH 3 3 + . CH 2 C Br
3 3
3 3
CH 3 CH 3 CH 3 CH 3
(CH ) COCl
3 3
. CH C Br BrCH C . BrCH C Cl + (CH ) CO .
2
2
2
3 3
CH 3 CH 3 CH 3
Entry 4 is an alkenyl migration that presumably occurs through a bicyclic radical.
CH 2 . .
.
The reaction in Entry 5 involves a phenyl migration. The rearranged radical undergoes
hydrogen atom elimination rather than the usual abstraction of bromine.
11.6. S 1 Substitution Processes
RN
The mechanistic description S RN 1 refers to a nucleophilic substitution via a radical
intermediate that proceeds by unimolecular decomposition of a radical anion derived
from the reactant. There are two families of such reactions that have been developed