Page 1065 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 1065
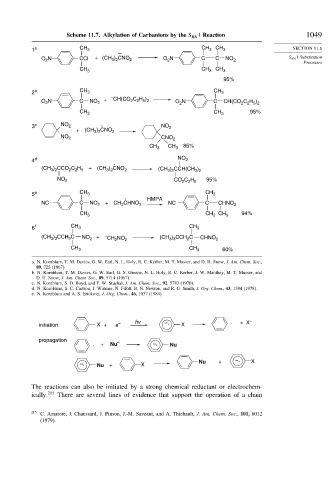
Scheme 11.7. Alkylation of Carbanions by the S RN 1 Reaction 1049
1 a CH 3 CH 3 CH 3 SECTION 11.6
–
O N CCl + (CH 3 ) 2 CNO 2 O N C C NO 2 S RN 1 Substitution
2
2
Processes
CH 3 CH 3 CH 3
95%
2 b CH 3 CH 3
– CH(CO C H )
O N C NO 2 + 2 2 5 2 O N C CH(CO C H )
2
2 2 5 2
2
CH 3 CH 3 95%
3 c NO 2 - NO 2
+ (CH ) CNO 2
3 2
NO 2 CNO 2
CH 3 CH 3 85%
4 d NO 2
-
(CH ) CCO C H + (CH ) CNO 2 (CH ) CCH(CH )
2 2 5
3 2
3 2
3 2
3 2
NO 2 CO C H 95%
2 2 5
5 e CH 3 CH 3
- HMPA
NC C NO 2 + CH CHNO 2 NC C CHNO 2
3
CH 3 CH CH 3 94%
3
6 f CH 3 CH 3
(CH ) CCH C NO 2 + – CH NO 2 (CH ) CCH C CHNO 2
2
3 3
2
3 3
2
CH 3 CH 3 60%
a. N. Kornblum, T. M. Davies, G. W. Earl, N. L. Holy, R. C. Kerber, M. T. Musser, and D. H. Snow, J. Am. Chem. Soc.,
89, 725 (1967).
b. N. Kornblum, T. M. Davies, G. W. Earl, G. S. Greene, N. L. Holy, R. C. Kerber, J. W. Manthey, M. T. Musser, and
D. H. Snow, J. Am. Chem. Soc., 89, 5714 (1967).
c. N. Kornblum, S. D. Boyd, and F. W. Stuchal, J. Am. Chem. Soc., 92, 5783 (1970).
d. N. Kornblum, S. C. Carlson, J. Widmer, N. Fifolt, B. N. Newton, and R. G. Smith, J. Org. Chem., 43, 1394 (1978).
e. N. Kornblum and A. S. Erickson, J. Org. Chem., 46, 1037 (1984).
initiation X + e – hv –. X . + X –
propagation
. + Nu – –. Nu
Nu + –. X
–. Nu + X
The reactions can also be initiated by a strong chemical reductant or electrochem-
ically. 215 There are several lines of evidence that support the operation of a chain
215
C. Amatore, J. Chaussard, J. Pinson, J.-M. Saveant, and A. Thiebault, J. Am. Chem. Soc., 101, 6012
(1979).