Page 1070 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 1070
1054 Larson and Cremer have explored another approach to dissecting BDE into
inherent and RSE effects. 228 There is a relationship between C−H BDE and the vibra-
CHAPTER 11 tional frequencies of the bonds. 229 Furthermore, the vibrations can be determined for
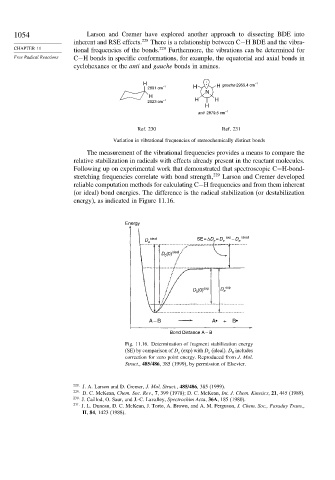
Free Radical Reactions C−H bonds in specific conformations, for example, the equatorial and axial bonds in
cyclohexanes or the anti and gauche bonds in amines.
.
H . H gauche 2955.4 cm –1
2891 cm –1 H
N
H
2923 cm –1 H H
H
–1
anti 2879.5 cm
Ref. 230 Ref. 231
Variation in vibrational frequencies of stereochemically distinct bonds
The measurement of the vibrational frequencies provides a means to compare the
relative stabilization in radicals with effects already present in the reactant molecules.
Following up on experimental work that demonstrated that spectroscopic C−H-bond-
stretching frequencies correlate with bond strength, 229 Larson and Cremer developed
reliable computation methods for calculating C−H frequencies and from them inherent
(or ideal) bond energies. The difference is the radical stabilization (or destabilization
energy), as indicated in Figure 11.16.
Energy
D e ideal SE = ΔD = D exp – D ideal
e
e
e
D (0) ideal
0
D (0) exp D e exp
0
A – B A• + B•
Bond Distance A – B
Fig. 11.16. Determination of fragment stabilization energy
(SE) by comparison of D e (exp) with D e (ideal). D 0 includes
correction for zero point energy. Reproduced from J. Mol.
Struct., 485/486, 385 (1999), by permission of Elsevier.
228 J. A. Larson and D. Cremer, J. Mol. Struct., 485/486, 385 (1999).
229
D. C. McKean, Chem. Soc. Rev., 7, 399 (1978); D. C. McKean, Int. J. Chem. Kinetics, 21, 445 (1989).
230 J. Caillod, O. Saur, and J.-C. Lavalley, Spectrochim Acta, 36A, 185 (1980).
231
J. L. Duncan, D. C. McKean, J. Torto, A. Brown, and A. M. Ferguson, J. Chem. Soc., Faraday Trans.,
II, 84, 1423 (1988).