Page 1071 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 1071
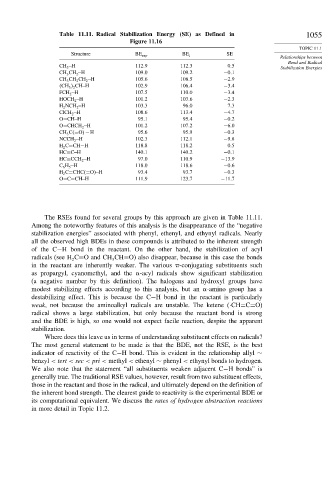
Table 11.11. Radical Stabilization Energy (SE) as Defined in 1055
Figure 11.16
TOPIC 11.1
Structure BE exp BE i SE
Relationships between
Bond and Radical
CH 3 −H 112 9 112 3 0 5
Stabilization Energies
CH 3 CH 2 −H 109 0 109 2 −0 1
CH 3 CH 2 CH 2 −H 105 6 108 5 −2 9
CH 3
2 CH−H 102 9 106 4 −3 4
FCH 2 −H 107 5 110 0 −3 4
HOCH 2 −H 101 2 103 6 −2 3
H 2 NCH 2 −H 103 3 96 0 7 3
ClCH 2 −H 108 6 113 4 −4 7
O=CH−H 95 1 95 4 −0 2
O=CHCH 2 −H 101 2 107 2 −6 0
CH 3 C =O
−H 95 6 95 9 −0 3
NCCH 2 −H 102 3 112 1 −9 8
H 2 C=CH−H 118 8 118 2 0 5
HC≡C−H 140 1 140 2 −0 1
HC≡CCH 2 −H 97 0 110 9 −13 9
C 6 H 5 −H 118 0 118 6 −0 6
H 2 C=CHC =O
−H 93 4 93 7 −0 3
O=C=CH−H 111 9 123 7 −11 7
The RSEs found for several groups by this approach are given in Table 11.11.
Among the noteworthy features of this analysis is the disappearance of the “negative
stabilization energies” associated with phenyl, ethenyl, and ethynyl radicals. Nearly
all the observed high BDEs in these compounds is attributed to the inherent strength
of the C−H bond in the reactant. On the other hand, the stabilization of acyl
radicals (see H C=O and CH CH=O) also disappear, because in this case the bonds
3
2
in the reactant are inherently weaker. The various -conjugating substituents such
as propargyl, cyanomethyl, and the -acyl radicals show significant stabilization
(a negative number by this definition). The halogens and hydroxyl groups have
modest stabilizing effects according to this analysis, but an -amino group has a
destabilizing effect. This is because the C−H bond in the reactant is particularly
weak, not because the aminoalkyl radicals are unstable. The ketene (·CH=C=O)
radical shows a large stabilization, but only because the reactant bond is strong
and the BDE is high, so one would not expect facile reaction, despite the apparent
stabilization.
Where does this leave us in terms of understanding substituent effects on radicals?
The most general statement to be made is that the BDE, not the RSE, is the best
indicator of reactivity of the C−H bond. This is evident in the relationship allyl ∼
benzyl < tert < sec < pri < methyl < ethenyl ∼ phenyl < ethynyl bonds to hydrogen.
We also note that the statement “all substituents weaken adjacent C−H bonds” is
generally true. The traditional RSE values, however, result from two substituent effects,
those in the reactant and those in the radical, and ultimately depend on the definition of
the inherent bond strength. The clearest guide to reactivity is the experimental BDE or
its computational equivalent. We discuss the rates of hydrogen abstraction reactions
in more detail in Topic 11.2.