Page 1075 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 1075
1059
ethyl ethane TOPIC 11.2
Structure-Reactivity
Relationships in
+
+ Hydrogen Abstraction
Reactions
propene
allyl
Transition state
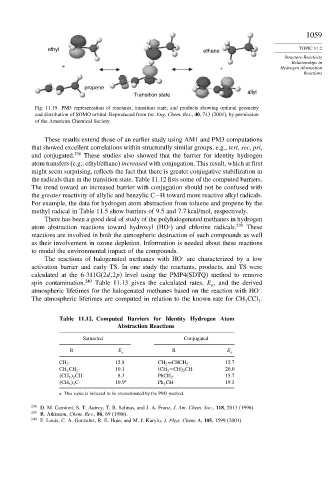
Fig. 11.19. PM3 representation of reactants, transition state, and products showing optimal geometry
and distribution of SOMO orbital. Reproduced from Int. Eng. Chem. Res., 40, 743 (2001), by permission
of the American Chemical Society.
These results extend those of an earlier study using AM1 and PM3 computations
that showed excellent correlations within structurally similar groups, e.g., tert, sec, pri,
and conjugated. 238 These studies also showed that the barrier for identity hydrogen
atom transfers (e.g., ethyl/ethane) increased with conjugation. This result, which at first
might seem surprising, reflects the fact that there is greater conjugative stabilization in
the radicals than in the transition state. Table 11.12 lists some of the computed barriers.
The trend toward an increased barrier with conjugation should not be confused with
the greater reactivity of allylic and benzylic C−H toward more reactive alkyl radicals.
For example, the data for hydrogen atom abstraction from toluene and propene by the
methyl radical in Table 11.5 show barriers of 9.5 and 7.7 kcal/mol, respectively.
There has been a good deal of study of the polyhalogenated methanes in hydrogen
. 239
atom abstraction reactions toward hydroxyl (HO ) and chlorine radicals. These
reactions are involved in both the atmospheric destruction of such compounds as well
as their involvement in ozone depletion. Information is needed about these reactions
to model the environmental impact of the compounds.
.
The reactions of halogenated methanes with HO are characterized by a low
activation barrier and early TS. In one study the reactants, products, and TS were
calculated at the 6-311G(2d 2p) level using the PMP4(SDTQ) method to remove
spin contamination. 240 Table 11.13 gives the calculated rates, E , and the derived
a
.
atmospheric lifetimes for the halogenated methanes based on the reaction with HO .
The atmospheric lifetimes are computed in relation to the known rate for CH CCl .
3
3
Table 11.12. Computed Barriers for Identity Hydrogen Atom
Abstraction Reactions
Saturated Conjugated
R E a R E a
CH 3 · 15.8 CH 2 =CHCH 2 · 15.7
CH 3 CH 2 · 10.1 (CH 2 =CH
2 CH· 20.0
CH 3
2 CH· 8.3 PhCH 2 · 15.7
CH 3
3 C· 10 9 a Ph 2 CH· 19.3
a. This value is believed to be overestimated by the PM3 method.
238
D. M. Camioni, S. T. Autrey, T. B. Salinas, and J. A. Franz, J. Am. Chem. Soc., 118, 2013 (1996).
239 R. Atkinson, Chem. Rev., 86, 69 (1986).
240
F. Louis, C. A. Gonzalez, R. E. Huie, and M. J. Kurylo, J. Phys. Chem. A, 105, 1599 (2001).