Page 1076 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 1076
1060 Table 11.13. Computed Rates, E , and Atmospheric Lifetimes for
a
Halomethanes a
CHAPTER 11
Free Radical Reactions Compound Number k 298 ×10 −15 E a /R Lifetime (years)
CH 3 F 2 21 1340 6 37
CH 3 Cl 5 189 1015 1 55
CH 3 Br 8 100 1190 2 47
3 23 1595 3 59
CH 2 F 2
CH 2 FCl 11 80 1160 1 68
6 433 510 0 43
CH 2 Cl 2
CH 2 ClBr 16 304 590 0 54
8 247 690 0 57
CH 2 Br 2
4 0 28 2640 397
CHF 3
CHF 2 Cl 12 9 1 1540 13 16
CHF 2 Br 15 15 1375 6 03
13 115 765 1 18
CHFCl 2
7 870 10 0 15
CHCl 3
CH 2 FBr 17 30 1440 1 88
17 100 825 0 49
CHFBr 2
CHFClBr 20 68 920 0 73
CHCl 2 Br 18 264 330 0 16
19 346 250 0 12
CHClBr 2
a. F. Louis, C. A. Gonzalez, R. E. Huie, and M. J. Kurylo, J. Phys. Chem. A, 105, 1599
(2001).
–28
m
f g h
–30
b c d
Ln k theory (298 K) –32 k a j
–34
l
–36
–38
–110 –100 –90 –80 –70 –60 –50 –40
H (ISO), kJ mol –1
Δ r
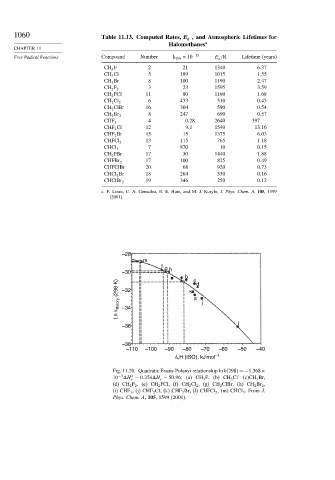
Fig. 11.20. Quadratic Evans-Polanyi relationship ln k 298
=−1 368×
−3
2
10 H − 0 354 H r − 50 96: (a) CH 3 F, (b) CH 3 Cl (c)CH 3 Br,
r
(d) CH 2 F 2 , (e) CH 2 FCl, (f) CH 2 Cl 2 , (g) CH 2 ClBr, (h) CH 2 Br 2 ,
(i) CHF 3 , (j) CHF 2 Cl, (k) CHF 2 Br, (l) CHFCl 2 , (m) CHCl 3 . From J.
Phys. Chem. A, 105, 1599 (2001).