Page 1081 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 1081
f. Among the products from heating 1,6-heptadiene with 1065
1-iodoperfluoropropane in the presence of AIBN are two saturated 1:1
PROBLEMS
adducts. Both adducts give the same product on dehydroiodination, and it
can be shown by spectroscopic means to contain a =CH unit. Indicate
2
structures for the two adducts and propose a mechanism for their formation.
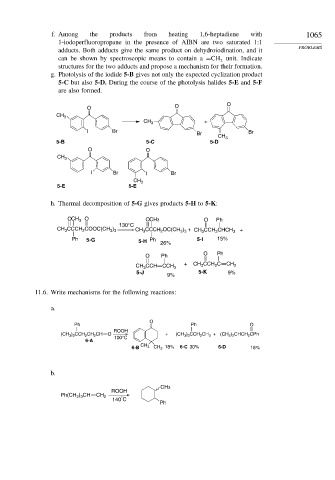
g. Photolysis of the iodide 5-B gives not only the expected cyclization product
5-C but also 5-D. During the course of the photolysis halides 5-E and 5-F
are also formed.
O O O
CH 3
+
CH 3
I Br Br Br
CH 3
5-B 5-C 5-D
O O
CH 3
I Br I Br
CH 3
5-E 5-E
h. Thermal decomposition of 5-G gives products 5-H to 5-K:
O CH 3 O O CH3 O Ph
130°C
CH CCCH COOC(CH ) CH CCCH OC(CH ) + CH CCH CHCH 3 +
3 3
3
2
2
2
3 3
3
3
Ph 5-G 5-H Ph 26% 5-I 15%
O Ph
O Ph
+ CH CCH C CH
CH CCH CCH 3 3 2 2
3
5-J 5-K 9%
9%
11.6. Write mechanisms for the following reactions:
a.
O
Ph Ph O
ROOH
(CH 3 ) 2 CCH 2 CH 2 CH O + (CH 3 ) 2 CCH 2 CH 3 + (CH 3 ) 2 CHCH 2 CPh
100°C
6-A
6-B CH 3 CH 3 18% 6-C 30% 6-D 18%
b.
CH3
ROOH
Ph(CH 2 ) 3 CH CH 2 °
140 C
Ph