Page 1084 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 1084
1068 chlorinating agents. Mechanistic study indicates that the reaction occurs by the
following chain sequence:
CHAPTER 11
Free Radical Reactions initiation R 2 N H R 2 NH +. + Cl .
+
Cl
propagation R 2 NH +. + CH 3 CH 2 (CH 2 ) n X + + CH 3 CH(CH 2 ) n X
.
R 2 NH 2
+
CH 3 CH(CH 2 ) n X + R 2 N H CH 3 CH(CH 2 ) n X + R 2 NH +.
.
Cl Cl
X = CO 2 CH 3 , CONH 2 , CH 2 OH
A very interesting feature of the reaction is that the chlorine is introduced
with high selectivity at the next-to-terminal position for molecules with n = 4
to 6. In contrast, chlorination in nonpolar solvents does not show comparable
selectivity. Rationalize these observations.
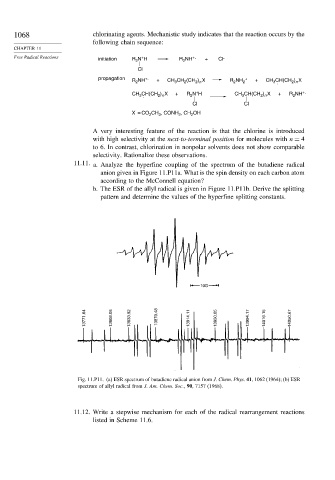
11.11. a. Analyze the hyperfine coupling of the spectrum of the butadiene radical
anion given in Figure 11.P11a. What is the spin density on each carbon atom
according to the McConnell equation?
b. The ESR of the allyl radical is given in Figure 11.P11b. Derive the splitting
pattern and determine the values of the hyperfine splitting constants.
10G
13771.64 13808.06 13833.92 13870.48 13914.11 13950.65 13994.17 14016.15 14050.67
Fig. 11.P11. (a) ESR spectrum of butadiene radical anion from J. Chem. Phys. 41, 1062 (1964); (b) ESR
spectrum of allyl radical from J. Am. Chem. Soc., 90, 7157 (1968).
11.12. Write a stepwise mechanism for each of the radical rearrangement reactions
listed in Scheme 11.6.