Page 1079 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 1079
P. Renaud and M. P. Sibi, Radicals in Organic Synthesis, Vol 1, Basic Principles, P. Renaud and M. P. Sibi, 1063
editors, Wiley-VCH, Weinheim, 2001.
PROBLEMS
Spectroscopic Methods
M. Bersohn and J. C. Baird, An Introduction to Electron Paramagnetic Resonance, W. A. Benjamin, New
York, 1966.
N. Hirota and H. Ohya-Nishiguchi, in Investigation of Rates and Mechanisms of Reactions, C. Bernasconi,
ed., Techniques of Chemistry, 4th Edition, Vol. VI, Part 2, Wiley-Interscience, New York, Chapter XI.
A. G. Lawler and H. R. Ward, in Determination of Organic Structures by Physical Methods, Vol 5, F. C.
Nachod and J. J. Zuckerman, eds., Academic Press, New York, 1973, Chap. 3.
F. Gerson and W. Huber, Electron Spin Resonance of Organic Radicals, Wiley-VCH, Weinheim, 2003.
Problems
(References for these problems will be found on page 1167.)
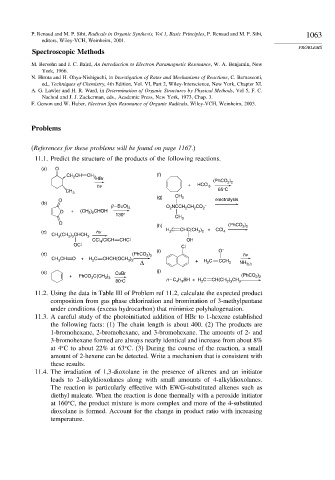
11.1. Predict the structure of the products of the following reactions.
(a) O
CH CH (f)
CH 2
2
HBr
(PhCO )
+ HCCl 2 2
hv 3
CH 3 65°C
(g) CH 3
O electrolysis
(b)
(t – BuO) O NCCH CH CO –
2 2 2 2 2
O + (CH ) CHOH
3 2
130°
CH
3
O (PhCO )
(h) 2 2
H C CHC(CH ) + CCl
(c) hv 2 3 2 4
CH (CH ) CHCH 3
2 3
3
CCl /ClCH CHCl OH
4
OCl Cl
(i) O –
(d) (PhCO ) hv
2 2
CH CH O + H C CHCH(OCH )
3
2
3 2
Δ + H C CCH 3 NH 3(l )
2
(e) CuBr (j)
+ PhCO C(CH ) (PhCO )
2 2
3 3 3 n – C H SH + H C CH(CH ) CH
80°C 4 9 2 2 3 3
11.2. Using the data in Table III of Problem ref 11.2, calculate the expected product
composition from gas phase chlorination and bromination of 3-methylpentane
under conditions (excess hydrocarbon) that minimize polyhalogenation.
11.3. A careful study of the photoinitiated addition of HBr to 1-hexene established
the following facts: (1) The chain length is about 400. (2) The products are
1-bromohexane, 2-bromohexane, and 3-bromohexane. The amounts of 2- and
3-bromohexane formed are always nearly identical and increase from about 8%
at 4 C to about 22% at 63 C. (3) During the course of the reaction, a small
amount of 2-hexene can be detected. Write a mechanism that is consistent with
these results.
11.4. The irradiation of 1,3-dioxolane in the presence of alkenes and an initiator
leads to 2-alkyldioxolanes along with small amounts of 4-alkyldioxolanes.
The reaction is particularly effective with EWG-substituted alkenes such as
diethyl maleate. When the reaction is done thermally with a peroxide initiator
at 160 C, the product mixture is more complex and more of the 4-substituted
dioxolane is formed. Account for the change in product ratio with increasing
temperature.