Page 1080 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 1080
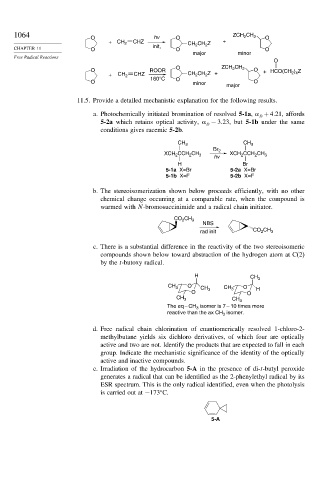
1064 O hv O ZCH 2 CH 2 O
+ CH 2 CHZ CH CH Z +
2
2
CHAPTER 11 O init, O O
major minor
Free Radical Reactions
O
O ZCH 2 CH
O ROOR 2 O ) Z
+ CH 2 CHZ CH 2 CH Z + + HCO(CH 2 4
2
O 160°C O minor O
major
11.5. Provide a detailed mechanistic explanation for the following results.
a. Photochemically initiated bromination of resolved 5-1a, +4 21, affords
D
5-2a which retains optical activity, − 3 23, but 5-1b under the same
D
conditions gives racemic 5-2b.
CH 3 CH 3
Br 2
XCH CCH CH 3 hv XCH CCH CH 3
2
2
2
2
H Br
5-1a X=Br 5-2a X=Br
5-1b X=F 5-2b X=F
b. The stereoisomerization shown below proceeds efficiently, with no other
chemical change occurring at a comparable rate, when the compound is
warmed with N-bromosuccinimide and a radical chain initiator.
CO CH 3
2
NBS
rad init CO CH 3
2
c. There is a substantial difference in the reactivity of the two stereoisomeric
compounds shown below toward abstraction of the hydrogen atom at C(2)
by the t-butoxy radical.
H
CH 3
CH 3 O CH CH 3 O H
O 3 O
CH 3 CH 3
The eq – CH isomer is 7 – 10 times more
3
reactive than the ax CH isomer.
3
d. Free radical chain chlorination of enantiomerically resolved 1-chloro-2-
methylbutane yields six dichloro derivatives, of which four are optically
active and two are not. Identify the products that are expected to fall in each
group. Indicate the mechanistic significance of the identity of the optically
active and inactive compounds.
e. Irradiation of the hydrocarbon 5-A in the presence of di-t-butyl peroxide
generates a radical that can be identified as the 2-phenylethyl radical by its
ESR spectrum. This is the only radical identified, even when the photolysis
is carried out at −173 C.
5-A