Page 1085 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 1085
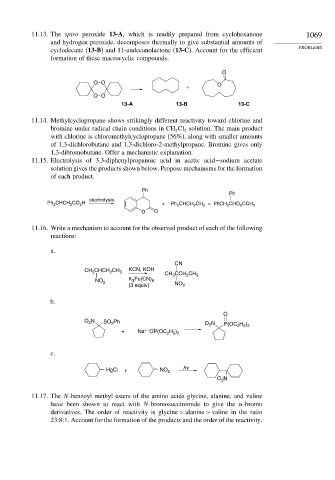
11.13. The spiro peroxide 13-A, which is readily prepared from cyclohexanone 1069
and hydrogen peroxide, decomposes thermally to give substantial amounts of
PROBLEMS
cyclodecane (13-B) and 11-undecanolactone (13-C). Account for the efficient
formation of these macrocyclic compounds.
O
O O O
+
O O
13-A 13-B 13-C
11.14. Methylcyclopropane shows strikingly different reactivity toward chlorine and
bromine under radical chain conditions in CH Cl solution. The main product
2
2
with chlorine is chloromethylcyclopropane (56%), along with smaller amounts
of 1,3-dichlorobutane and 1,3-dichloro-2-methylpropane. Bromine gives only
1,3-dibromobutane. Offer a mechanistic explanation.
11.15. Electrolysis of 3,3-diphenylpropanoic acid in acetic acid−sodium acetate
solution gives the products shown below. Propose mechanisms for the formation
of each product.
Ph
Ph
electrolysis
Ph 2 CHCH 2 CO 2 H + Ph 2 CHCH 2 CH 3 + PhCH 2 CHO 2 CCH 3
O O
11.16. Write a mechanism to account for the observed product of each of the following
reactions:
a.
CN
CH CHCH CH 3 KCN, KOH
2
3
2
CH 3 CCH CH 3
3
NO 2 K Fe(CN) 6
(3 equiv) NO 2
b.
O
O N SO Ph O N P(OC H )
2
2
2
2 5 2
+–
+ Na OP(OC H )
2 5 2
c.
HgCl + – NO 2 hv
O N
2
11.17. The N-benzoyl methyl esters of the amino acids glycine, alanine, and valine
have been shown to react with N-bromosuccinimide to give the -bromo
derivatives. The order of reactivity is glycine > alanine > valine in the ratio
23:8:1. Account for the formation of the products and the order of the reactivity.