Page 1087 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 1087
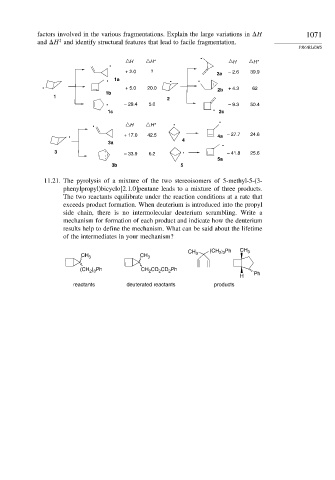
factors involved in the various fragmentations. Explain the large variations in H 1071
‡
and H and identify structural features that lead to facile fragmentation.
PROBLEMS
.
H H* H H*
.
+ 2.0 ? – 2.6 39.9
2a
. 1a . .
. + 5.0 20.0 2b + 4.3 62
1b
1 2
. – 29.4 5.0 – 9.3 50.4
.
1c 2c
.
. H H* .
. + 17.0 42.5 4a – 27.7 24.6
4
3a .
3 . – 41.8 25.6
. – 33.9 6.2
5a
3b 5
11.21. The pyrolysis of a mixture of the two stereoisomers of 5-methyl-5-(3-
phenylpropyl)bicyclo[2.1.0]pentane leads to a mixture of three products.
The two reactants equilibrate under the reaction conditions at a rate that
exceeds product formation. When deuterium is introduced into the propyl
side chain, there is no intermolecular deuterium scrambling. Write a
mechanism for formation of each product and indicate how the deuterium
results help to define the mechanism. What can be said about the lifetime
of the intermediates in your mechanism?
CH (CH ) 3 Ph CH 3
2
CH 3 CH 3 3
(CH ) Ph CH CD CD Ph
2
2
2
2 3
H Ph
reactants deuterated reactants products