Page 1082 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 1082
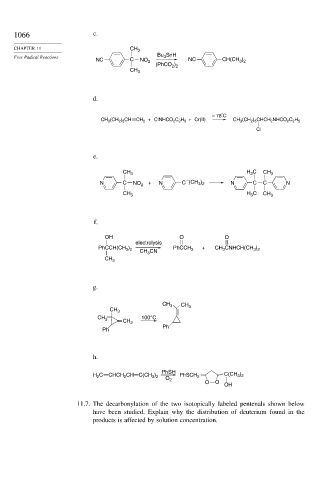
1066 c.
CHAPTER 11 CH 3
Bu SnH
Free Radical Reactions 3
NC C NO 2 NC CH(CH )
3 2
(PhCO )
2 2
CH 3
d.
°
– 78 C
CH 3 (CH 2 ) 5 CH CH 2 + ClNHCO 2 C 2 H 5 + Cr(II) CH 3 (CH 2 ) 5 CHCH 2 NHCO 2 C 2 H 5
Cl
e.
CH 3 H C CH 3
3
–
N C NO 2 + N C (CH ) N C C N
3 2
CH 3 H 3 C CH 3
f.
OH O O
electrolysis
PhCCH(CH ) PhCCH + CH 3 CNHCH(CH )
3 2
CH CN 3 3 2
3
CH 3
g.
CH 3 CH
CH 3 3
CH 3 CH 100°C
2
Ph
Ph
h.
PhSH
H C CHCH CH C(CH ) PhSCH 2 C(CH )
3 2
2
2
3 2
O 2
O O
OH
11.7. The decarbonylation of the two isotopically labeled pentenals shown below
have been studied. Explain why the distribution of deuterium found in the
products is affected by solution concentration.