Page 1083 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 1083
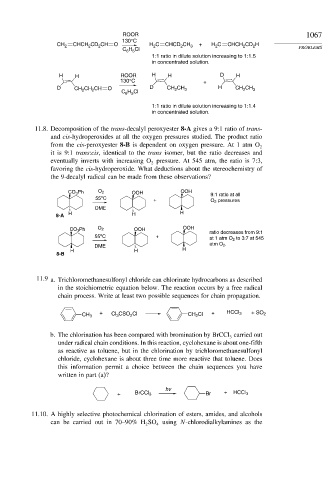
ROOR 1067
130°C
CH 2 CHCH CD CH O H C CHCD CH 3 + H C CHCH CD H
2
2
2
2
2
2
2
C H Cl PROBLEMS
6 5
1:1 ratio in dilute solution increasing to 1:1.5
in concentrated solution.
H H ROOR H H D H
130°C +
D CH CH CH O D CH CH 3 H CH CH 3
2
2
2
2
H Cl
C 6 5
1:1 ratio in dilute solution increasing to 1:1.4
in concentrated solution.
11.8. Decomposition of the trans-decalyl peroxyester 8-A gives a 9:1 ratio of trans-
and cis-hydroperoxides at all the oxygen pressures studied. The product ratio
from the cis-peroxyester 8-B is dependent on oxygen pressure. At 1 atm O 2
it is 9:1 trans:cis, identical to the trans isomer, but the ratio decreases and
eventually inverts with increasing O pressure. At 545 atm, the ratio is 7:3,
2
favoring the cis-hydroperoxide. What deductions about the stereochemistry of
the 9-decalyl radical can be made from these observations?
CO 3 Ph O 2 OOH OOH 9:1 ratio at all
o
55 C + O 2 pressures
DME
8-A H H H
CO 3 Ph O 2 OOH OOH ratio decreases from 9:1
o
55 C + at 1 atm O 2 to 3:7 at 545
atm O 2 .
DME
H H H
8-B
11.9 a. Trichloromethanesulfonyl chloride can chlorinate hydrocarbons as described
in the stoichiometric equation below. The reaction occurs by a free radical
chain process. Write at least two possible sequences for chain propagation.
+ Cl CSO Cl + HCCl 3 + SO 2
CH 3 3 2 CH 2 Cl
b. The chlorination has been compared with bromination by BrCCl carried out
3
under radical chain conditions. In this reaction, cyclohexane is about one-fifth
as reactive as toluene, but in the chlorination by trichloromethanesulfonyl
chloride, cyclohexane is about three time more reactive that toluene. Does
this information permit a choice between the chain sequences you have
written in part (a)?
hv
+ BrCCl 3 Br + HCCl 3
11.10. A highly selective photochemical chlorination of esters, amides, and alcohols
can be carried out in 70–90% H SO using N-chlorodialkylamines as the
4
2