Page 1091 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 1091
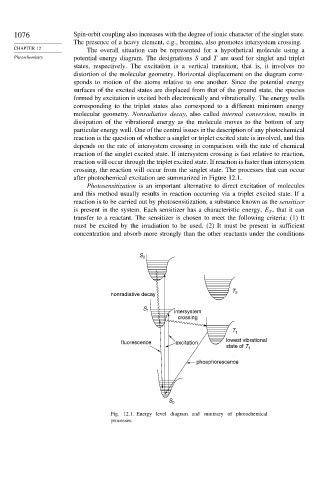
1076 Spin-orbit coupling also increases with the degree of ionic character of the singlet state.
The presence of a heavy element, e.g., bromine, also promotes intersystem crossing.
CHAPTER 12 The overall situation can be represented for a hypothetical molecule using a
Photochemistry potential energy diagram. The designations S and T are used for singlet and triplet
states, respectively. The excitation is a vertical transition; that is, it involves no
distortion of the molecular geometry. Horizontal displacement on the diagram corre-
sponds to motion of the atoms relative to one another. Since the potential energy
surfaces of the excited states are displaced from that of the ground state, the species
formed by excitation is excited both electronically and vibrationally. The energy wells
corresponding to the triplet states also correspond to a different minimum energy
molecular geometry. Nonradiative decay, also called internal conversion, results in
dissipation of the vibrational energy as the molecule moves to the bottom of any
particular energy well. One of the central issues in the description of any photochemical
reaction is the question of whether a singlet or triplet excited state is involved, and this
depends on the rate of intersystem crossing in comparison with the rate of chemical
reaction of the singlet excited state. If intersystem crossing is fast relative to reaction,
reaction will occur through the triplet excited state. If reaction is faster than intersystem
crossing, the reaction will occur from the singlet state. The processes that can occur
after photochemical excitation are summarized in Figure 12.1.
Photosensitization is an important alternative to direct excitation of molecules
and this method usually results in reaction occurring via a triplet excited state. If a
reaction is to be carried out by photosensitization, a substance known as the sensitizer
is present in the system. Each sensitizer has a characteristic energy, E , that it can
T
transfer to a reactant. The sensitizer is chosen to meet the following criteria: (1) It
must be excited by the irradiation to be used. (2) It must be present in sufficient
concentration and absorb more strongly than the other reactants under the conditions
S 2
T
nonradiative decay 2
S 1 intersystem
crossing
T 1
lowest vibrational
fluorescence excitation
state of T 1
phosphorescence
S 0
Fig. 12.1. Energy level diagram and summary of photochemical
processes.