Page 1094 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 1094
The mechanisms of photochemical reactions can be presented at several levels 1079
of detail. The most basic level is to recognize the unpairing/re-pairing sequence that
is associated with bond breaking and bond forming. These processes can be further SECTION 12.1
described by depicting the orbitals that are involved. Just as in thermal reactions, orbital General Principles
symmetry and/or stereoelectronic effects can be recognized in this way. Photochemical
reactions can also be described by potential energy diagrams, similar to those we
have used for thermal reactions. For a photochemical reaction, the diagram represents
transitions between the excited structures and aims to trace the path from excitation
to photoproduct. As for thermal reactions, the path depicted is the minimum energy
path across a potential energy surface. Photochemical reactions, however, can involve
several excited states, each with its own potential energy surface, so there are several
energy plots representing these surfaces. Two-dimensional representations can depict
progress in one structural change, such as a twist about a bond or a bond breaking.
Alternatively, the reaction progress may be viewed as a composite of all the structural
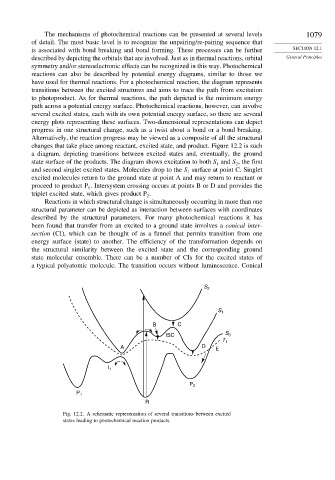
changes that take place among reactant, excited state, and product. Figure 12.2 is such
a diagram, depicting transitions between excited states and, eventually, the ground
state surface of the products. The diagram shows excitation to both S and S , the first
2
1
and second singlet excited states. Molecules drop to the S surface at point C. Singlet
1
excited molecules return to the ground state at point A and may return to reactant or
proceed to product P . Intersystem crossing occurs at points B or D and provides the
1
triplet excited state, which gives product P .
2
Reactions in which structural change is simultaneously occurring in more than one
structural parameter can be depicted as interaction between surfaces with coordinates
described by the structural parameters. For many photochemical reactions it has
been found that transfer from an excited to a ground state involves a conical inter-
section (CI), which can be thought of as a funnel that permits transition from one
energy surface (state) to another. The efficiency of the transformation depends on
the structural similarity between the excited state and the corresponding ground
state molecular ensemble. There can be a number of CIs for the excited states of
a typical polyatomic molecule. The transition occurs without luminescence. Conical
S 2
S 1
B C
S
ISC 0
T 1
A D E
I 1
P 2
P 1
R
Fig. 12.2. A schematic representation of several transitions between excited
states leading to photochemical reaction products.