Page 1099 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 1099
1084 is believed to be planar with a barrier to rotation of the double bond with respect to the
10
ring of at least 3–4 kcal/mol. The decay time for the S styrene is in the nanosecond
1
CHAPTER 12 11
range, which is considerably longer than for ethene. The S excited state of styrene
2
Photochemistry is a zwitterionic structure similar to the S state for ethene. In this case, as might be
1
expected, the terminal methylene group is pyramidalized, with the cationic character
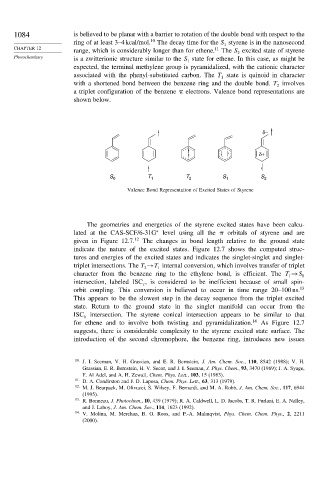
associated with the phenyl-substituted carbon. The T state is quinoid in character
1
with a shortened bond between the benzene ring and the double bond. T involves
2
a triplet configuration of the benzene electrons. Valence bond representations are
shown below.
δ–
δ+
S 0 T 1 T 2 S 1 S 2
Valence Bond Representation of Excited States of Styrene
The geometries and energetics of the styrene excited states have been calcu-
∗
lated at the CAS-SCF/6-31G level using all the orbitals of styrene and are
given in Figure 12.7. 12 The changes in bond length relative to the ground state
indicate the nature of the excited states. Figure 12.7 shows the computed struc-
tures and energies of the excited states and indicates the singlet-singlet and singlet-
triplet intersections. The T →T internal conversion, which involves transfer of triplet
2 1
character from the benzene ring to the ethylene bond, is efficient. The T →S 0
1
intersection, labeled ISC , is considered to be inefficient because of small spin-
c
orbit coupling. This conversion is believed to occur in time range 20–100 ns. 13
This appears to be the slowest step in the decay sequence from the triplet excited
state. Return to the ground state in the singlet manifold can occur from the
ISC intersection. The styrene conical intersection appears to be similar to that
b
for ethene and to involve both twisting and pyramidalization. 14 As Figure 12.7
suggests, there is considerable complexity to the styrene excited state surface. The
introduction of the second chromophore, the benzene ring, introduces new issues
10
J. I. Seeman, V. H. Grassian, and E. R. Bernstein, J. Am. Chem. Soc., 110, 8542 (1988); V. H.
Grassian, E. R. Bernstein, H. V. Secor, and J. I. Seeman, J. Phys. Chem., 93, 3470 (1989); J. A. Syage,
F. Al Adel, and A. H. Zewail, Chem. Phys. Lett., 103, 15 (1983).
11 D. A. Condirston and J. D. Laposa, Chem. Phys. Lett., 63, 313 (1979).
12
M. J. Bearpack, M. Olivucci, S. Wilsey, F. Bernardi, and M. A. Robb, J. Am. Chem. Soc., 117, 6944
(1995).
13 R. Bonneau, J. Photochem., 10, 439 (1979); R. A. Caldwell, L. D. Jacobs, T. R. Furlani, E. A. Nalley,
and J. Laboy, J. Am. Chem. Soc., 114, 1623 (1992).
14
V. Molina, M. Merchan, B. O. Roos, and P.-A. Malmqvist, Phys. Chem. Chem. Phys., 2, 2211
(2000).