Page 1101 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 1101
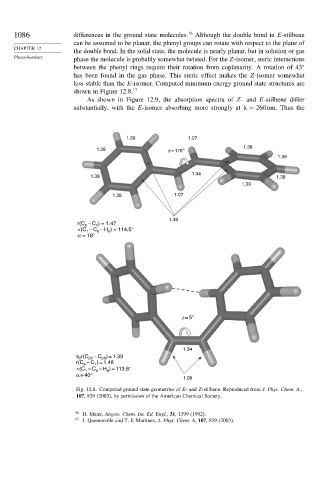
1086 differences in the ground state molecules. 16 Although the double bond in E-stilbene
can be assumed to be planar, the phenyl groups can rotate with respect to the plane of
CHAPTER 12
the double bond. In the solid state, the molecule is nearly planar, but in solution or gas
Photochemistry
phase the molecule is probably somewhat twisted. For the Z-isomer, steric interactions
between the phenyl rings require their rotation from coplanarity. A rotation of 43
has been found in the gas phase. This steric effect makes the Z-isomer somewhat
less stable than the E-isomer. Computed minimum energy ground state structures are
shown in Figure 12.8. 17
As shown in Figure 12.9, the absorption spectra of Z- and E-stilbene differ
substantially, with the E-isomer absorbing more strongly at > 260nm. Thus the
1.39 1.07
1.39 φ = 178° 1.38
1.39
1.34
1.39 1.39
1.39
1.38 1.07
1.40
r(C – C ) = 1.47
e 1
<(C – C – H ) = 114.5°
1 e e
α = 18°
φ = 5°
1.34
s r(C ph ph
– C ) = 1.39
0
r(C – C ) = 1.48
e 1
– C – H ) = 113.8°
<(C 1 e e
α = 40°
1.08
Fig. 12.8. Computed ground state geometries of E- and Z-stilbene. Reproduced from J. Phys. Chem. A.,
107, 829 (2003), by permission of the American Chemical Society.
16 H. Meier, Angew. Chem. Int. Ed. Engl., 31, 1399 (1992).
17
J. Quenneville and T. J. Martinez, J. Phys. Chem. A, 107, 829 (2003).