Page 1102 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 1102
1087
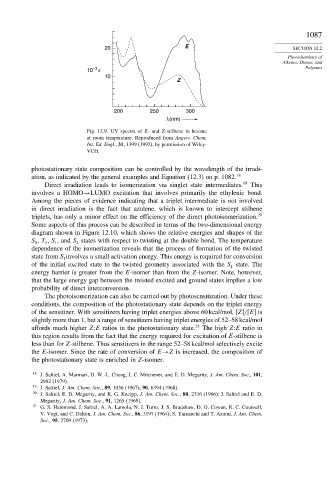
20 E SECTION 12.2
Photochemistry of
Alkenes, Dienes, and
10 –3 ε Polyenes
10
Z
200 250 300
λ(nm)
Fig. 12.9. UV spectra of E- and Z-stilbene in hexane
at room temperature. Reproduced from Angew. Chem.
Int. Ed. Engl., 31, 1399 (1992), by permission of Wiley-
VCH.
photostationary state composition can be controlled by the wavelength of the irradi-
ation, as indicated by the general examples and Equation (12.3) on p. 1082. 18
Direct irradiation leads to isomerization via singlet state intermediates. 19 This
involves a HOMO→LUMO excitation that involves primarily the ethylenic bond.
Among the pieces of evidence indicating that a triplet intermediate is not involved
in direct irradiation is the fact that azulene, which is known to intercept stilbene
triplets, has only a minor effect on the efficiency of the direct photoisomerization. 20
Some aspects of this process can be described in terms of the two-dimensional energy
diagram shown in Figure 12.10, which shows the relative energies and shapes of the
S , T , S , and S states with respect to twisting at the double bond. The temperature
0
1
1
2
dependence of the isomerization reveals that the process of formation of the twisted
state from S involves a small activation energy. This energy is required for conversion
1
of the initial excited state to the twisted geometry associated with the S state. The
1
energy barrier is greater from the E-isomer than from the Z-isomer. Note, however,
that the large energy gap between the twisted excited and ground states implies a low
probability of direct interconversion.
The photoisomerization can also be carried out by photosensitization. Under these
conditions, the composition of the photostationary state depends on the triplet energy
of the sensitizer. With sensitizers having triplet energies above 60 kcal/mol, Z
/ E
is
slightly more than 1, but a range of sensitizers having triplet energies of 52–58 kcal/mol
affords much higher Z:E ratios in the photostationary state. 21 The high Z:E ratio in
this region results from the fact that the energy required for excitation of E-stilbene is
less than for Z-stilbene. Thus sensitizers in the range 52–58 kcal/mol selectively excite
the E-isomer. Since the rate of conversion of E→Z is increased, the composition of
the photostationary state is enriched in Z-isomer.
18 J. Saltiel, A. Marinari, D. W.-L. Chang, J. C. Mitchener, and E. D. Megarity, J. Am. Chem. Soc., 101,
2982 (1979).
19
J. Saltiel, J. Am. Chem. Soc., 89, 1036 (1967); 90, 6394 (1968).
20 J. Saltiel, E. D. Megarity, and K. G. Kneipp, J. Am. Chem. Soc., 88, 2336 (1966); J. Saltiel and E. D.
Megarity, J. Am. Chem. Soc., 91, 1265 (1969).
21
G. S. Hammond, J. Saltiel, A. A. Lamola, N. J. Turro, J. S. Bradshaw, D. O. Cowan, R. C. Counsell,
V. Vogt, and C. Dalton, J. Am. Chem. Soc., 86, 3197 (1964); S. Yamauchi and T. Azumi, J. Am. Chem.
Soc., 95, 2709 (1973).