Page 1103 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 1103
1088
Photosensitizer a E T Z E
CHAPTER 12
Acetophenone 73 6 1 2
Photochemistry
Benzophenone 68 5 1 2
2-Acetylnaphthalene 59 3 2 6
1-Naphthyl phenyl ketone 57 5 2 8
Benzil 53 7 4 6
Fluorenone 52 3 8 3
Pyrene 48 7 4 6
3-Acetylpyrene 45 2 8
a. From J. Am. Chem. Soc., 86, 3197 (1964).
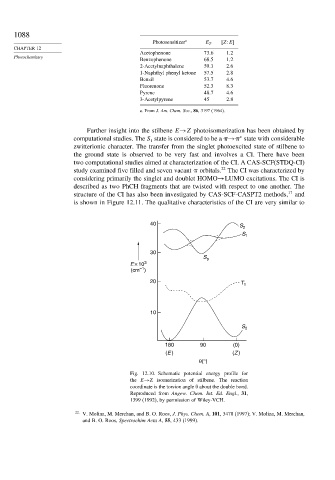
Further insight into the stilbene E→Z photoisomerization has been obtained by
∗
computational studies. The S state is considered to be a → state with considerable
1
zwitterionic character. The transfer from the singlet photoexcited state of stilbene to
the ground state is observed to be very fast and involves a CI. There have been
two computational studies aimed at characterization of the CI. A CAS-SCF(STDQ-CI)
22
study examined five filled and seven vacant orbitals. The CI was characterized by
considering primarily the singlet and doublet HOMO→LUMO excitations. The CI is
described as two PhCH fragments that are twisted with respect to one another. The
structure of the CI has also been investigated by CAS-SCF-CASPT2 methods, 17 and
is shown in Figure 12.11. The qualitative characteristics of the CI are very similar to
40 S 2
S 1
30
S p
E × 10 3
–1
(cm )
20 T 1
10
S 0
180 90 (0)
(E ) (Z )
θ(°)
Fig. 12.10. Schematic potential energy profile for
the E→Z isomerization of stilbene. The reaction
coordinate is the torsion angle about the double bond.
Reproduced from Angew. Chem. Int. Ed. Engl., 31,
1399 (1992), by permission of Wiley-VCH.
22
V. Molina, M. Merchan, and B. O. Roos, J. Phys. Chem. A, 101, 3478 (1997); V. Molina, M. Merchan,
and B. O. Roos, Spectrochim Acta A, 55, 433 (1999).