Page 1104 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 1104
1089
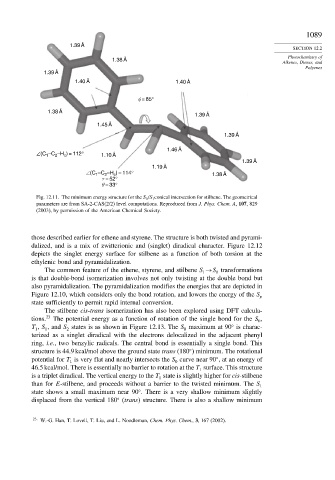
1.39 Å
SECTION 12.2
Photochemistry of
1.38 Å
Alkenes, Dienes, and
Polyenes
1.39 Å
1.40 Å 1.40 Å
φ = 85°
1.38 Å
1.39 Å
1.45 Å
1.39 Å
1.46 Å
–C –H ) = 112°
∠(C 1 2 c 1.10 Å
1.39 Å
1.19 Å
∠(C –C –H ) = 114° 1.38 Å
2
c
1
τ = 52°
θ = 33°
Fig. 12.11. The minimum energy structure for the S 0 /S 1 conical intersection for stilbene. The geometrical
parameters are from SA-2-CAS(2/2) level computations. Reproduced from J. Phys. Chem. A, 107, 829
(2003), by permission of the American Chemical Society.
those described earlier for ethene and styrene. The structure is both twisted and pyrami-
dalized, and is a mix of zwitterionic and (singlet) diradical character. Figure 12.12
depicts the singlet energy surface for stilbene as a function of both torsion at the
ethylenic bond and pyramidalization.
The common feature of the ethene, styrene, and stilbene S →S transformations
1
0
is that double-bond isomerization involves not only twisting at the double bond but
also pyramidalization. The pyramidalization modifies the energies that are depicted in
Figure 12.10, which considers only the bond rotation, and lowers the energy of the S p
state sufficiently to permit rapid internal conversion.
The stilbene cis-trans isomerization has also been explored using DFT calcula-
tions. 23 The potential energy as a function of rotation of the single bond for the S ,
0
T , S , and S states is as shown in Figure 12.13. The S maximum at 90 is charac-
1 1 2 0
terized as a singlet diradical with the electrons delocalized in the adjacent phenyl
ring, i.e., two benzylic radicals. The central bond is essentially a single bond. This
structure is 44.9 kcal/mol above the ground state trans (180 ) minimum. The rotational
potential for T is very flat and nearly intersects the S curve near 90 , at an energy of
1
0
46.5 kcal/mol. There is essentially no barrier to rotation at the T surface. This structure
1
is a triplet diradical. The vertical energy to the T state is slightly higher for cis-stilbene
1
than for E-stilbene, and proceeds without a barrier to the twisted minimum. The S 1
state shows a small maximum near 90 . There is a very shallow minimum slightly
displaced from the vertical 180 (trans) structure. There is also a shallow minimum
23
W.-G. Han, T. Lovell, T. Liu, and L. Noodleman, Chem. Phys. Chem., 3, 167 (2002).