Page 1109 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 1109
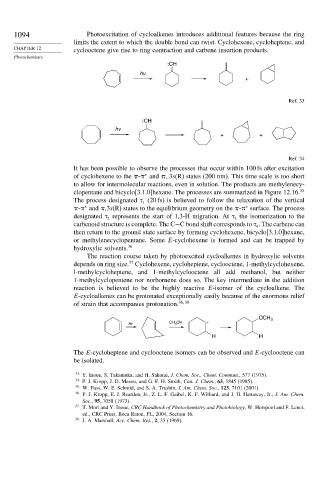
1094 Photoexcitation of cycloalkenes introduces additional features because the ring
limits the extent to which the double bond can twist. Cyclohexene, cycloheptene, and
CHAPTER 12 cyclooctene give rise to ring contraction and carbene insertion products.
Photochemistry
:CH
hv
+
Ref. 33
:CH
hv
+ +
Ref. 34
It has been possible to observe the processes that occur within 100 fs after excitation
∗
of cyclohexene to the - and ,3s(R) states (200 nm). This time scale is too short
to allow for intermolecular reactions, even in solution. The products are methylenecy-
clopentane and bicyclo[3.1.0]hexane. The processes are summarized in Figure 12.16. 35
The process designated (20 fs) is believed to follow the relaxation of the vertical
1
∗
- and ,3s(R) states to the equilibrium geometry on the - surface. The process
∗
designated represents the start of 1,3-H migration. At the isomerization to the
2 3
carbenoid structure is complete. The C−C bond shift corresponds to . The carbene can
4
then return to the ground state surface by forming cyclohexene, bicyclo[3.1.0]hexane,
or methylenecyclopentane. Some E-cyclohexene is formed and can be trapped by
hydroxylic solvents. 36
The reaction course taken by photoexcited cycloalkenes in hydroxylic solvents
37
depends on ring size. Cyclohexene, cycloheptene, cyclooctene, 1-methylcyclohexene,
l-methylcycloheptene, and 1-methylcyclooctene all add methanol, but neither
1-methylcyclopentene nor norbornene does so. The key intermediate in the addition
reaction is believed to be the highly reactive E-isomer of the cycloalkene. The
E-cycloalkenes can be protonated exceptionally easily because of the enormous relief
of strain that accompanies protonation. 36 38
OCH 3
hv CH OH +
3
H H
The E-cycloheptene and cyclooctene isomers can be observed and E-cyclooctene can
be isolated.
33
Y. Inoue, S. Takamuka, and H. Sakurai, J. Chem. Soc., Chem. Commun., 577 (1975).
34 P. J. Kropp, J. D. Mason, and G. F. H. Smith, Can. J. Chem., 63, 1845 (1985).
35
W. Fuss, W. E. Schmid, and S. A. Trushin, J. Am. Chem. Soc., 123, 7101 (2001).
36
P. J. Kropp, E. J. Reardon, Jr., Z. L. F. Gaibel, K. F. Williard, and J. H. Hattaway, Jr., J. Am. Chem.
Soc., 95, 7058 (1973).
37 T. Mori and Y. Inoue, CRC Handbook of Photochemistry and Photobiology, W. Horspool and F. Lenci,
ed., CRC Press, Boca Raton, FL, 2004, Section 16.
38
J. A. Marshall, Acc. Chem. Res., 2, 33 (1969).