Page 1110 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 1110
R S 1 1095
S 2
τ SECTION 12.2
τ 1 1
Photochemistry of
τ 2 Alkenes, Dienes, and
Polyenes
CH CH
τ 3
200 nm
CH
CH τ 4
S 0 H C
(CH)
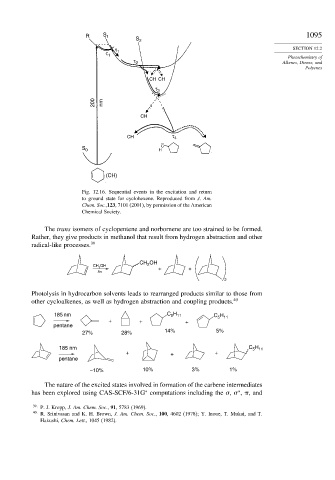
Fig. 12.16. Sequential events in the excitation and return
to ground state for cyclohexene. Reproduced from J. Am.
Chem. Soc.,123, 7101 (2001), by permission of the American
Chemical Society.
The trans isomers of cyclopentene and norbornene are too strained to be formed.
Rather, they give products in methanol that result from hydrogen abstraction and other
radical-like processes. 39
CH OH
CH OH 2
3 + +
h ν
2
Photolysis in hydrocarbon solvents leads to rearranged products similar to those from
other cycloalkenes, as well as hydrogen abstraction and coupling products. 40
185 nm C H C H
5 11
5 11
+ + +
pentane
27% 28% 14% 5%
185 nm C H
5 11
+ + +
pentane
~10% 10% 3% 1%
The nature of the excited states involved in formation of the carbene intermediates
∗
∗
has been explored using CAS-SCF/6-31G computations including the , , , and
39 P. J. Kropp, J. Am. Chem. Soc., 91, 5783 (1969).
40
R. Srinivasan and K. H. Brown, J. Am. Chem. Soc., 100, 4602 (1978); Y. Inoue, T. Mukai, and T.
Hakushi, Chem. Lett., 1045 (1982).