Page 1108 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 1108
H 1093
2 2 2
R 3 1 3 1 R 3 1
. + . + RCH C . CH 2 SECTION 12.2
2
. . .
1,3-alkyl R H H Photochemistry of
shift 2,1-hydrogen Alkenes, Dienes, and
3,2-shift and 3,1-hydrogen Polyenes
1,3-coupling shift shift to carbene
RCH CCH 3
: 2
R
R CH 3
R
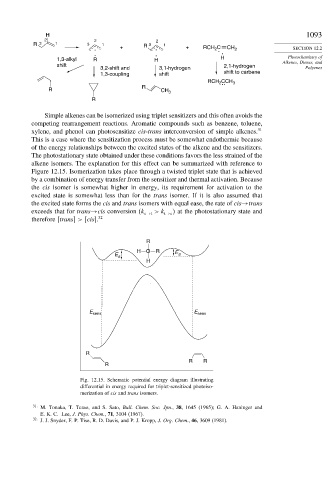
Simple alkenes can be isomerized using triplet sensitizers and this often avoids the
competing rearrangement reactions. Aromatic compounds such as benzene, toluene,
xylene, and phenol can photosensitize cis-trans interconversion of simple alkenes. 31
This is a case where the sensitization process must be somewhat endothermic because
of the energy relationships between the excited states of the alkene and the sensitizers.
The photostationary state obtained under these conditions favors the less strained of the
alkene isomers. The explanation for this effect can be summarized with reference to
Figure 12.15. Isomerization takes place through a twisted triplet state that is achieved
by a combination of energy transfer from the sensitizer and thermal activation. Because
the cis isomer is somewhat higher in energy, its requirement for activation to the
excited state is somewhat less than for the trans isomer. If it is also assumed that
the excited state forms the cis and trans isomers with equal ease, the rate of cis→trans
exceeds that for trans→cis conversion (k c→t >k t→c at the photostationary state and
therefore [trans] > [cis]. 32
R
H O R E
E a a
H
E sens E sens
R
R R
R
Fig. 12.15. Schematic potential energy diagram illustrating
differential in energy required for triplet-sensitized photoiso-
merization of cis and trans isomers.
31 M. Tonaka, T. Terao, and S. Sato, Bull. Chem. Soc. Jpn., 38, 1645 (1965); G. A. Haninger and
E. K. C. Lee, J. Phys. Chem., 71, 3104 (1967).
32
J. J. Snyder, F. P. Tise, R. D. Davis, and P. J. Kropp, J. Org. Chem., 46, 3609 (1981).