Page 1111 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 1111
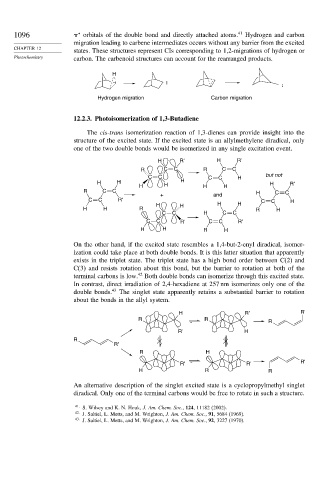
1096 orbitals of the double bond and directly attached atoms. 41 Hydrogen and carbon
∗
migration leading to carbene intermediates occurs without any barrier from the excited
CHAPTER 12
states. These structures represent CIs corresponding to 1,2-migrations of hydrogen or
Photochemistry carbon. The carbenoid structures can account for the rearranged products.
H
:
:
Hydrogen migration Carbon migration
12.2.3. Photoisomerization of 1,3-Butadiene
The cis-trans isomerization reaction of 1,3-dienes can provide insight into the
structure of the excited state. If the excited state is an allylmethylene diradical, only
one of the two double bonds would be isomerized in any single excitation event.
H R' H R'
R C C R C C
C C C C H but not
H H H H R'
H H H H
R C C H C C
+ and
C C R' C C H
H H H H
H H R R H
C C H C C
C C R' C C R'
H H R H
On the other hand, if the excited state resembles a 1,4-but-2-enyl diradical, isomer-
ization could take place at both double bonds. It is this latter situation that apparently
exists in the triplet state. The triplet state has a high bond order between C(2) and
C(3) and resists rotation about this bond, but the barrier to rotation at both of the
42
terminal carbons is low. Both double bonds can isomerize through this excited state.
In contrast, direct irradiation of 2,4-hexadiene at 257 nm isomerizes only one of the
double bonds. 43 The singlet state apparently retains a substantial barrier to rotation
about the bonds in the allyl system.
H R' R'
R R
R
R' H
R
R'
R H
R' R' R'
H R R
An alternative description of the singlet excited state is a cyclopropylmethyl singlet
diradical. Only one of the terminal carbons would be free to rotate in such a structure.
41
S. Wilsey and K. N. Houk, J. Am. Chem. Soc., 124, 11182 (2002).
42 J. Saltiel, L. Metts, and M. Wrighton, J. Am. Chem. Soc., 91, 5684 (1969).
43
J. Saltiel, L. Metts, and M. Wrighton, J. Am. Chem. Soc., 92, 3227 (1970).