Page 1116 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 1116
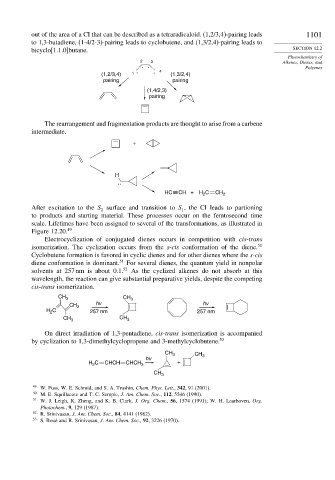
out of the area of a CI that can be described as a tetraradicaloid. (1,2/3,4)-pairing leads 1101
to 1,3-butadiene, (1-4/2-3)-pairing leads to cyclobutene, and (1,3/2,4)-pairing leads to
bicyclo[1.1.0]butane. SECTION 12.2
Photochemistry of
2 3 Alkenes, Dienes, and
. . Polyenes
(1,2/3,4) 1 . . 4 (1,3/2,4)
pairing pairing
(1,4/2,3)
pairing
The rearrangement and fragmentation products are thought to arise from a carbene
intermediate.
+
H
:
HC CH + H 2 C CH 2
After excitation to the S surface and transition to S , the CI leads to partioning
2 1
to products and starting material. These processes occur on the femtosecond time
scale. Lifetimes have been assigned to several of the transformations, as illustrated in
Figure 12.20. 49
Electrocyclization of conjugated dienes occurs in competition with cis-trans
isomerization. The cyclization occurs from the s-cis conformation of the diene. 50
Cyclobutene formation is favored in cyclic dienes and for other dienes where the s-cis
diene conformation is dominant. 51 For several dienes, the quantum yield in nonpolar
solvents at 257 nm is about 0.1. 52 As the cyclized alkenes do not absorb at this
wavelength, the reaction can give substantial preparative yields, despite the competing
cis-trans isomerization.
CH 3 CH 3
CH 2 hv hv
H C 257 nm 257 nm
2
CH 3 CH 3
On direct irradiation of 1,3-pentadiene, cis-trans isomerization is accompanied
by cyclization to 1,3-dimethylcyclopropene and 3-methylcyclobutene. 53
CH 3 CH 3
hv
H 2 C CHCH CHCH 3 +
CH 3
49
W. Fuss, W. E. Schmid, and S. A. Trushin, Chem. Phys. Lett., 342, 91 (2001).
50
M. E. Squillacote and T. C. Semple, J. Am. Chem. Soc., 112, 5546 (1990).
51 W. J. Leigh, K. Zheng, and K. B. Clark, J. Org. Chem., 56, 1574 (1991); W. H. Laarhoven, Org.
Photochem., 9, 129 (1987).
52 R. Srinivasan, J. Am. Chem. Soc., 84, 4141 (1982).
53
S. Boué and R. Srinivasan, J. Am. Chem. Soc., 92, 3226 (1970).