Page 1118 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 1118
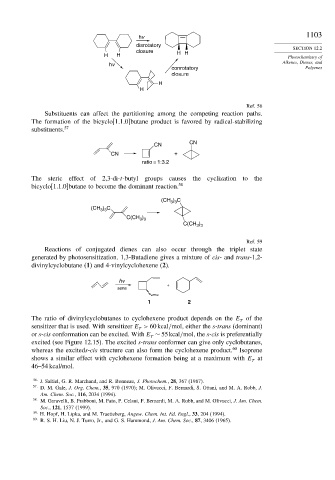
hv 1103
disrotatory
SECTION 12.2
closure H H
H H Photochemistry of
Alkenes, Dienes, and
hv
conrotatory Polyenes
closure
H
H
Ref. 56
Substituents can affect the partitioning among the competing reaction paths.
The formation of the bicyclo[1.1.0]butane product is favored by radical-stabilizing
substituents. 57
CN
CN
CN +
ratio = 1:3.2
The steric effect of 2,3-di-t-butyl groups causes the cyclization to the
bicyclo[1.1.0]butane to become the dominant reaction. 58
(CH ) C
3 3
(CH ) C
3 3
C(CH )
3 3
C(CH )
3 3
Ref. 59
Reactions of conjugated dienes can also occur through the triplet state
generated by photosensitization. 1,3-Butadiene gives a mixture of cis- and trans-1,2-
divinylcyclobutane (1) and 4-vinylcyclohexene (2).
hv
+
sens
1 2
The ratio of divinylcyclobutanes to cyclohexene product depends on the E of the
T
sensitizer that is used. With sensitizer E > 60kcal/mol, either the s-trans (dominant)
T
or s-cis conformation can be excited. With E ∼ 55kcal/mol, the s-cis is preferentially
T
excited (see Figure 12.15). The excited s-trans conformer can give only cyclobutanes,
whereas the exciteds-cis structure can also form the cyclohexene product. 60 Isoprene
shows a similar effect with cyclohexene formation being at a maximum with E at
T
46–54 kcal/mol.
56
J. Saltiel, G. R. Marchand, and R. Bonneau, J. Photochem., 28, 367 (1987).
57
D. M. Gale, J. Org. Chem., 35, 970 (1970); M. Olivucci, F. Bernardi, S. Ottani, and M. A. Robb, J.
Am. Chem. Soc., 116, 2034 (1994).
58 M. Garavelli, B. Frabboni, M. Fato, P. Celani, F. Bernardi, M. A. Robb, and M. Olivucci, J. Am. Chem.
Soc., 121, 1537 (1999).
59 H. Hopf, H. Lipka, and M. Traetteberg, Angew. Chem. Int. Ed. Engl., 33, 204 (1994).
60
R. S. H. Liu, N. J. Turro, Jr., and G. S. Hammond, J. Am. Chem. Soc., 87, 3406 (1965).