Page 1122 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 1122
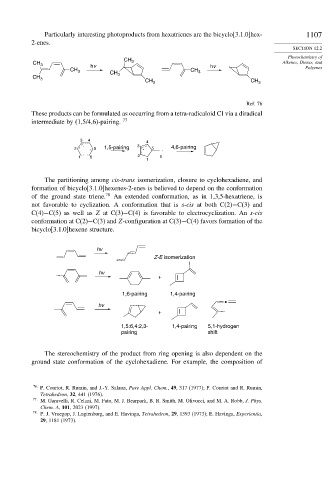
Particularly interesting photoproducts from hexatrienes are the bicyclo[3.1.0]hex- 1107
2-enes.
SECTION 12.2
Photochemistry of
CH 3 hv CH 3 hv Alkenes, Dienes, and
CH 3 CH CH 3 Polyenes
CH 3 3
CH 3 CH 3
Ref. 76
These products can be formulated as occurring from a tetra-radicaloid CI via a diradical
intermediate by (1,5/4,6)-pairing. 77
3 4 4
. 3
2 . 5 1,5-pairing . 5 . 4,6-pairing
. .
1 6 2 6
1
The partitioning among cis-trans isomerization, closure to cyclohexadiene, and
formation of bicyclo[3.1.0]hexenes-2-enes is believed to depend on the conformation
of the ground state triene. 78 An extended conformation, as in 1,3,5-hexatriene, is
not favorable to cyclization. A conformation that is s-cis at both C(2)−C(3) and
C(4)−C(5) as well as Z at C(3)−C(4) is favorable to electrocyclization. An s-cis
conformation at C(2)−C(3) and Z-configuration at C(3)−C(4) favors formation of the
bicyclo[3.1.0]hexene structure.
hv
Z-E isomerization
hv
+
1,6-pairing 1,4-pairing
hv
+
1,5:6,4:2,3- 1,4-pairing 5,1-hydrogen
pairing shift
The stereochemistry of the product from ring opening is also dependent on the
ground state conformation of the cyclohexadiene. For example, the composition of
76
P. Courtot, R. Rumin, and J.-Y. Salaun, Pure Appl. Chem., 49, 317 (1977); P. Courtot and R. Rumin,
Tetrahedron, 32, 441 (1976).
77 M. Garavelli, R. Celani, M. Fato, M. J. Bearpark, B. R. Smith, M. Olivucci, and M. A. Robb, J. Phys.
Chem. A, 101, 2023 (1997).
78
P. J. Vroegop, J. Lugtenburg, and E. Havinga, Tetrahedron, 29, 1393 (1973); E. Havinga, Experientia,
29, 1181 (1973).