Page 1124 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 1124
indicate the importance of reactant conformation in influencing the reaction outcome. 1109
By contrast, the ring opening of cyclobutenes is more complex. In Topic 12.1 we take
a look at the computational interpretation of these results. SECTION 12.2
Photochemistry of
Alkenes, Dienes, and
Polyenes
12.2.6. Photochemical Cycloaddition Reactions
As described on p. 1098, the original orbital symmetry interpretation of alkene
[2 +2 ] cycloaddition was in terms of a suprafacial transition structure with a rectan-
gular geometry. This arrangement leads to a photochemical process that is allowed by
orbital symmetry criteria. Early experimental work also provided examples of stere-
ospecific [2 +2 ] cycloadditions, lending support to a concerted reaction path. For
example, dimerization of Z-2-butene gives two products that retain cis-methyl groups.
The adducts from E-2-butene have trans-methyl groups. 82 This establishes that the
configuration is retained at both alkene double bonds during the formation of the
dimers.
CH 3 CH 3 CH 3 CH 3
CH 3 CH 3 +
CH 3 CH 3 CH 3 CH 3
CH 3 CH CH CH
CH 3 3 3 3
+
CH 3
CH 3 CH 3 CH 3 CH 3
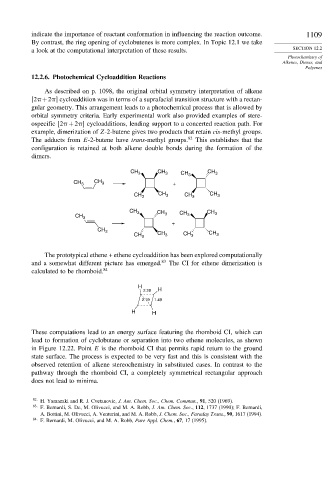
The prototypical ethene + ethene cycloaddition has been explored computationally
and a somewhat different picture has emerged. 83 The CI for ethene dimerization is
calculated to be rhomboid. 84
H
2.28 H
2.19 1.49
H H
These computations lead to an energy surface featuring the rhomboid CI, which can
lead to formation of cyclobutane or separation into two ethene molecules, as shown
in Figure 12.22. Point E is the rhomboid CI that permits rapid return to the ground
state surface. The process is expected to be very fast and this is consistent with the
observed retention of alkene stereochemistry in substituted cases. In contrast to the
pathway through the rhomboid CI, a completely symmetrical rectangular approach
does not lead to minima.
82
H. Yamazaki and R. J. Cvetanovic, J. Am. Chem. Soc., Chem. Commun., 91, 520 (1969).
83 F. Bernardi, S. De, M. Olivucci, and M. A. Robb, J. Am. Chem. Soc., 112, 1737 (1990); F. Bernardi,
A. Bottini, M. Olivucci, A. Venturini, and M. A. Robb, J. Chem. Soc., Faraday Trans., 90, 1617 (1994).
84
F. Bernardi, M. Olivucci, and M. A. Robb, Pure Appl. Chem., 67, 17 (1995).