Page 1120 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 1120
66
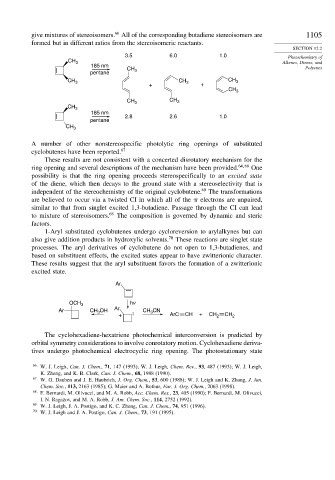
give mixtures of stereoisomers. All of the corresponding butadiene stereoisomers are 1105
formed but in different ratios from the stereoisomeric reactants.
SECTION 12.2
3.5 6.0 1.0 Photochemistry of
CH 3 Alkenes, Dienes, and
185 nm Polyenes
CH 3
pentane
CH 3 CH 3 CH 3
+ +
CH 3
CH 3 CH 3
CH 3
185 nm
2.8 2.6 1.0
pentane
CH 3
A number of other nonstereospecific photolytic ring openings of substituted
cyclobutenes have been reported. 67
These results are not consistent with a concerted disrotatory mechanism for the
ring opening and several descriptions of the mechanism have been provided. 64 68 One
possibility is that the ring opening proceeds stereospecifically to an excited state
of the diene, which then decays to the ground state with a stereoselectivity that is
independent of the stereochemistry of the original cyclobutene. 69 The transformations
are believed to occur via a twisted CI in which all of the electrons are unpaired,
similar to that from singlet excited 1,3-butadiene. Passage through the CI can lead
to mixture of stereoisomers. 68 The composition is governed by dynamic and steric
factors.
1-Aryl substituted cyclobutenes undergo cycloreversion to arylalkynes but can
also give addition products in hydroxylic solvents. 70 These reactions are singlet state
processes. The aryl derivatives of cyclobutene do not open to 1,3-butadienes, and
based on substituent effects, the excited states appear to have zwitterionic character.
These results suggest that the aryl substituent favors the formation of a zwitterionic
excited state.
Ar
OCH 3 hv
Ar CH OH Ar CH CN
3
3
+ : ArC CH + CH 2 CH 2
The cyclohexadiene-hexatriene photochemical interconversion is predicted by
orbital symmetry considerations to involve conrotatory motion. Cyclohexadiene deriva-
tives undergo photochemical electrocyclic ring opening. The photostationary state
66 W. J. Leigh, Can. J. Chem., 71, 147 (1993); W. J. Leigh, Chem. Rev., 93, 487 (1993); W. J. Leigh,
K. Zheng, and K. B. Clark, Can. J. Chem., 68, 1988 (1990).
67
W. G. Dauben and J. E. Haubrich, J. Org. Chem., 53, 600 (1988); W. J. Leigh and K. Zhang, J. Am.
Chem. Soc., 113, 2163 (1985); G. Maier and A. Bothur, Eur. J. Org. Chem., 2063 (1998).
68
F. Bernardi, M. Olivucci, and M. A. Robb, Acc. Chem. Res., 23, 405 (1990); F. Bernardi, M. Olivucci,
I. N. Ragazos, and M. A. Robb, J. Am. Chem. Soc., 114, 2752 (1992).
69 W. J. Leigh, J. A. Postigo, and K. C. Zheng, Can. J. Chem., 74, 951 (1996).
70
W. J. Leigh and J. A. Postigo, Can. J. Chem., 73, 191 (1995).