Page 1121 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 1121
1106 favors the triene, and the reaction is highly stereospecific. 71 E,Z,E−Octa-2,4,6-triene
gives trans-5,6-dimethylcyclohexene. 72
CHAPTER 12
Photochemistry
CH 3
hv
CH 3 CH 3 CH 3 CH 3 CH 3
Ring opening of more highly substituted cyclohexadienes also follows the Woodward-
Hoffmann rules.
The conrotatory electrocyclic ring opening of 1,3-cyclohexadiene can be observed
by several ultrafast techniques. 73 It is believed that the first step after excitation is
attainment of a planar geometry, which occurs within 10 –11 s. 74 The observations are
consistent with an excitation followed by return to the ground state via a CI that
permits formation of either reactant or Z-1,3,5-hexatriene. The lifetimes of the two
excited states are both shorter than 100 fs, and the product is formed within 200 fs. 75
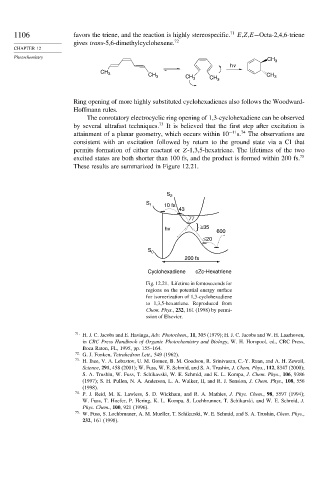
These results are summarized in Figure 12.21.
S 2
S 1 10 fs
43
77
} ≥35
hv
600
≤20
S 0
200 fs
Cyclohexadiene cZc-Hexatriene
Fig. 12.21. Lifetime in femtoseconds for
regions on the potential energy surface
for isomerization of 1,3-cyclohexadiene
to 1,3,5-hexatriene. Reproduced from
Chem. Phys., 232, 161 (1998) by permi-
ssion of Elsevier.
71
H. J. C. Jacobs and E. Havinga, Adv. Photochem., 11, 305 (1979); H. J. C. Jacobs and W. H. Laarhoven,
in CRC Press Handbook of Organic Photochemistry and Biology, W. H. Horspool, ed., CRC Press,
Boca Raton, FL, 1995, pp. 155–164.
72 G. J. Fonken, Tetrahedron Lett., 549 (1962).
73
H. Ihee, V. A. Lebastov, U. M. Gomez, B. M. Goodson, R. Srinivasan, C.-Y. Ruan, and A. H. Zewail,
Science, 291, 458 (2001); W. Fuss, W. E. Schmid, and S. A. Trushin, J. Chem. Phys., 112, 8347 (2000);
S. A. Trushin, W. Fuss, T. Schikavski, W. E. Schmid, and K. L. Kompa, J. Chem. Phys., 106, 9386
(1997); S. H. Pullen, N. A. Anderson, L. A. Walker, II, and R. J. Sension, J. Chem. Phys., 108, 556
(1998).
74 P. J. Reid, M. K. Lawless, S. D. Wickham, and R. A. Mathies, J. Phys. Chem., 98, 5597 (1994);
W. Fuss, T. Hoefer, P. Hering, K. L. Kompa, S. Lochbrunner, T. Schikarski, and W. E. Schmid, J.
Phys. Chem., 100, 921 (1996).
75
W. Fuss, S. Lochbrunner, A. M. Mueller, T. Schikarski, W. E. Schmid, and S. A. Trushin, Chem. Phys.,
232, 161 (1998).