Page 1126 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 1126
Under low intensity, the concentration of the E-isomer is too low to permit this 1111
reaction.
SECTION 12.2
H Ph H Ph Photochemistry of
Alkenes, Dienes, and
Polyenes
H + H
H H
Intramolecular cycloaddition is observed for 1,4-cyclooctadiene. 87
The most useful intermolecular [2 +2 ] cycloadditions from a synthetic point of
view involve alkenes and cyclic ,ß-unsaturated carbonyl compounds. These reactions
are discussed in more detail in Section 6.3.2 of Part B. Scheme 12.1 lists some
examples of photochemical cycloaddition and electrocyclic reactions of the type that
are consistent with the predictions of orbital symmetry considerations.
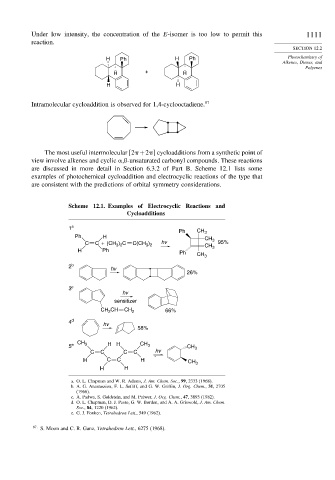
Scheme 12.1. Examples of Electrocyclic Reactions and
Cycloadditions
1 a
Ph CH 3
Ph H CH
) C
C C + (CH 3 2 C(CH ) hv CH 3 95%
3 2
H Ph Ph CH 3 3
2 b
hv
26%
3 c
hv
sensitizer
CH CH CH 2 66%
2
4 d hv
58%
CH CH
5 e 3 H H 3 CH 3
C C C C hv
H C C H CH 3
H H
a. O. L. Chapman and W. R. Adams, J. Am. Chem. Soc., 99, 2333 (1968).
b. A. G. Anastassiou, F. L. Setliff, and G. W. Griffin, J. Org. Chem., 31, 2705
(1966).
c. A. Padwa, S. Goldstein, and M. Pulwer, J. Org. Chem., 47, 3893 (1982).
d. O. L. Chapman, D. J. Pasto, G. W. Borden, and A. A. Griswold, J. Am. Chem.
Soc., 84, 1220 (1962).
e. G. J. Fonken, Tetrahedron Lett., 549 (1962).
87
S. Moon and C. R. Ganz, Tetrahedron Lett., 6275 (1968).