Page 1129 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 1129
1114 observed stereochemistry of the reaction. It remains to be seen if this mechanism
applies to the more highly substituted systems that have been studied experimentally.
CHAPTER 12
Photochemistry
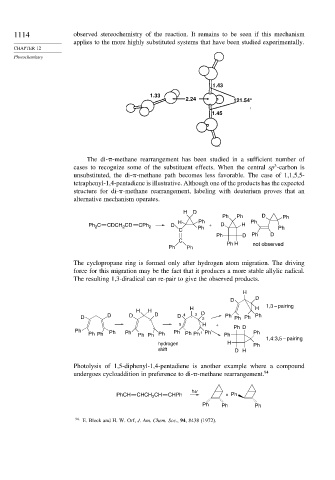
1.43
1.33
2.24 121.54°
1.45
The di- -methane rearrangement has been studied in a sufficient number of
3
cases to recognize some of the substituent effects. When the central sp -carbon is
unsubstituted, the di- -methane path becomes less favorable. The case of 1,1,5,5-
tetraphenyl-1,4-pentadiene is illustrative. Although one of the products has the expected
structure for di- -methane rearrangement, labeling with deuterium proves that an
alternative mechanism operates.
H D
Ph Ph D Ph
H Ph Ph
Ph C CDCH CD CPh 2 D Ph + D H Ph
2
2
C
Ph D Ph D
C Ph H
Ph Ph not observed
The cyclopropane ring is formed only after hydrogen atom migration. The driving
force for this migration may be the fact that it produces a more stable allylic radical.
The resulting 1,3-diradical can re-pair to give the observed products.
H
D D
H H 1,3 – pairing
H H D
D D D . D D 4 . 3 2 Ph Ph Ph Ph
5 H +
. . Ph D
Ph Ph Ph Ph Ph
Ph Ph Ph Ph Ph Ph Ph Ph 1 Ph
1,4:3,5 – pairing
hydrogen H Ph
shift D H
Photolysis of 1,5-diphenyl-1,4-pentadiene is another example where a compound
undergoes cycloaddition in preference to di- -methane rearrangement. 94
hv
PhCH CHCH CH CHPh + Ph
2
Ph Ph Ph
94
E. Block and H. W. Orf, J. Am. Chem. Soc., 94, 8438 (1972).