Page 1131 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 1131
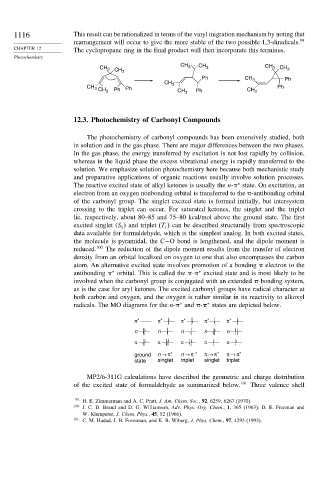
1116 This result can be rationalized in terms of the vinyl migration mechanism by noting that
rearrangement will occur to give the more stable of the two possible 1,3-diradicals. 99
CHAPTER 12 The cyclopropane ring in the final product will then incorporate this terminus.
Photochemistry
CH . CH
CH 3 CH 3 CH 3 3 CH 3
3
Ph CH Ph
CH 3 . 3
CH 3 CH 3 Ph Ph CH 3 Ph CH 3 Ph
12.3. Photochemistry of Carbonyl Compounds
The photochemistry of carbonyl compounds has been extensively studied, both
in solution and in the gas phase. There are major differences between the two phases.
In the gas phase, the energy transferred by excitation is not lost rapidly by collision,
whereas in the liquid phase the excess vibrational energy is rapidly transferred to the
solution. We emphasize solution photochemistry here because both mechanistic study
and preparative applications of organic reactions usually involve solution processes.
The reactive excited state of alkyl ketones is usually the n- state. On excitation, an
∗
electron from an oxygen nonbonding orbital is transferred to the -antibonding orbital
of the carbonyl group. The singlet excited state is formed initially, but intersystem
crossing to the triplet can occur. For saturated ketones, the singlet and the triplet
lie, respectively, about 80–85 and 75–80 kcal/mol above the ground state. The first
excited singlet (S and triplet (T can be described structurally from spectroscopic
1
1
data available for formaldehyde, which is the simplest analog. In both excited states,
the molecule is pyramidal, the C−O bond is lengthened, and the dipole moment is
reduced. 100 The reduction of the dipole moment results from the transfer of electron
density from an orbital localized on oxygen to one that also encompasses the carbon
atom. An alternative excited state involves promotion of a bonding electron to the
antibonding orbital. This is called the - excited state and is most likely to be
∗
∗
involved when the carbonyl group is conjugated with an extended -bonding system,
as is the case for aryl ketones. The excited carbonyl groups have radical character at
both carbon and oxygen, and the oxygen is rather similar in its reactivity to alkoxyl
∗
radicals. The MO diagrams for the n- and - states are depicted below.
∗
π ∗ π ∗ π ∗ π ∗ π ∗
n n n n n
π π π π π
ground n → π ∗ n → π ∗ π → π ∗ π → π ∗
state singlet triplet singlet triplet
MP2/6-311G calculations have described the geometric and charge distribution
of the excited state of formaldehyde as summarized below. 101 Three valence shell
99
H. E. Zimmerman and A. C. Pratt, J. Am. Chem. Soc., 92, 6259, 6267 (1970).
100 J. C. D. Brand and D. G. Williamson, Adv. Phys. Org. Chem., 1, 365 (1963); D. E. Freeman and
W. Klemperer, J. Chem. Phys., 45, 52 (1966).
101
C. M. Hadad, J. B. Foresman, and K. B. Wiberg, J. Phys. Chem., 97, 4293 (1993).