Page 1135 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 1135
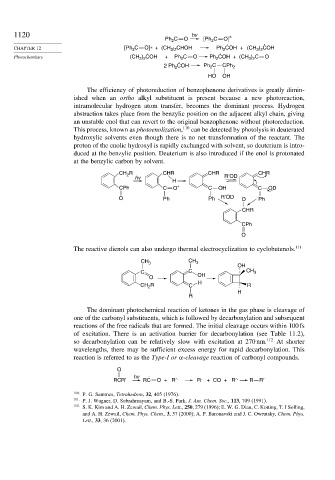
1120 hv ∗
Ph C O [Ph C O] . .
2
2
CHAPTER 12 [Ph 2 C O]∗ + (CH ) 2 CHOH Ph COH + (CH ) COH
3
3 2
2
. .
Photochemistry (CH ) COH + Ph 2 C O Ph 2 COH + (CH 3 ) 2 C O
3 2
.
2 Ph COH Ph C CPh 2
2
2
HO OH
The efficiency of photoreduction of benzophenone derivatives is greatly dimin-
ished when an ortho alkyl substituent is present because a new photoreaction,
intramolecular hydrogen atom transfer, becomes the dominant process. Hydrogen
abstraction takes place from the benzylic position on the adjacent alkyl chain, giving
an unstable enol that can revert to the original benzophenone without photoreduction.
This process, known as photoenolization, 110 can be detected by photolysis in deuterated
hydroxylic solvents even though there is no net transformation of the reactant. The
proton of the enolic hydroxyl is rapidly exchanged with solvent, so deuterium is intro-
duced at the benzylic position. Deuterium is also introduced if the enol is protonated
at the benzylic carbon by solvent.
CH R CHR CHR CHR
2
hv R′OD
H
CPh C O ∗ C OH C OD
O Ph Ph R′OD D Ph
CHR
CPh
O
The reactive dienols can also undergo thermal electrocyclization to cyclobutenols. 111
CH
CH 3 3
OH
C C OH CH 3
O
H
CH R C R
2
H
R
The dominant photochemical reaction of ketones in the gas phase is cleavage of
one of the carbonyl substituents, which is followed by decarbonylation and subsequent
reactions of the free radicals that are formed. The initial cleavage occurs within 100 fs
of excitation. There is an activation barrier for decarbonylation (see Table 11.2),
so decarbonylation can be relatively slow with excitation at 270 nm. 112 At shorter
wavelengths, there may be sufficient excess energy for rapid decarbonylation. This
reaction is referred to as the Type-I or -cleavage reaction of carbonyl compounds.
O
.
RCR′ hv RC . O + R′ . R + CO + R′ . R R′
110
P. G. Sammes, Tetrahedron, 32, 405 (1976).
111 P. J. Wagner, D. Subrahmayam, and B.-S. Park, J. Am. Chem. Soc., 113, 709 (1991).
112
S. K. Kim and A. H. Zewail, Chem. Phys. Lett., 250, 279 (1996); E. W. G. Diau, C. Kotting, T. I Solling,
and A. H. Zewail, Chem. Phys. Chem., 3, 57 (2000); A. P. Baronavski and J. C. Owrutsky, Chem. Phys.
Lett., 33, 36 (2001).