Page 1139 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 1139
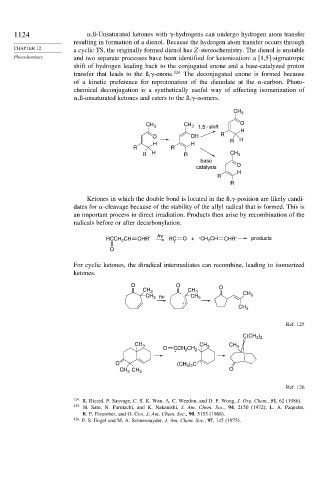
1124 ,ß-Unsaturated ketones with -hydrogens can undergo hydrogen atom transfer
resulting in formation of a dienol. Because the hydrogen atom transfer occurs through
CHAPTER 12 a cyclic TS, the originally formed dienol has Z-stereochemistry. The dienol is unstable
Photochemistry and two separate processes have been identified for ketonization: a [1,5]-sigmatropic
shift of hydrogen leading back to the conjugated enone and a base-catalyzed proton
transfer that leads to the ß, -enone. 124 The deconjugated enone is formed because
of a kinetic preference for reprotonation of the dienolate at the -carbon. Photo-
chemical deconjugation is a synthetically useful way of effecting isomerization of
,ß-unsaturated ketones and esters to the ß, -isomers.
CH 3
CH 3 CH 3 1,5 - shift O
H
O OH R
R H
H H
R R
R H R CH 3
base
catalysis O
H
R
R
Ketones in which the double bond is located in the ß, -position are likely candi-
dates for -cleavage because of the stability of the allyl radical that is formed. This is
an important process in direct irradiation. Products then arise by recombination of the
radicals before or after decarbonylation.
.
RCCH CH CHR′ hv RC . O + CH CH CHR′ products
2
2
O
For cyclic ketones, the diradical intermediates can recombine, leading to isomerized
ketones.
O O O
. 3
CH 3 CH
CH 3 hv CH 3 CH 3
.
CH 3
Ref. 125
C(CH )
3 2
CH 3 CH 3 CH 3
O CCH 2 CH 2
.
O (CH ) C .
3 2
CH 3 CH 3 O
Ref. 126
124 R. Ricard, P. Sauvage, C. S. K. Wan, A. C. Weedon, and D. F. Wong, J. Org. Chem., 51, 62 (1986).
125 H. Sato, N. Furutachi, and K. Nakanishi, J. Am. Chem. Soc., 94, 2150 (1972); L. A. Paquette,
R. F. Eizember, and O. Cox, J. Am. Chem. Soc., 90, 5153 (1968).
126
P. S. Engel and M. A. Schnexnayder, J. Am. Chem. Soc., 97, 145 (1975).