Page 1140 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 1140
12.3.2. Cycloaddition and Rearrangement Reactions of Cyclic Unsaturated 1125
Ketones
SECTION 12.3
Cyclic ,
-unsaturated ketones present a rich array of photochemical reactions, Photochemistry of
Carbonyl Compounds
some of which are of considerable synthetic value (see Part B, Section 6.3.2.2).
Generally, noncyclic enones relax rapidly by cis-trans interconversion and do not
undergo intermolecular photochemicial reactions. One useful reaction of cyclic enones
is photochemical addition of alkenes. 127 The reaction involves the - triplet excited
∗
state and 1,4-diradical intermediates. 128 Both the regiochemistry and stereochemistry
of the reaction are determined by the properties of the diradical intermediate. It appears
that initial bonding can occur at either C(2) or C(3) of the excited enone system.
The alkene reacts at its less-substituted terminus, generating the more stable radical.
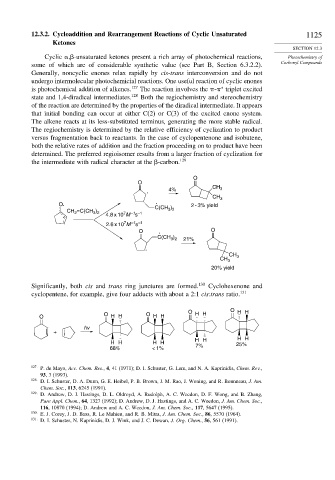
The regiochemistry is determined by the relative efficiency of cyclization to product
versus fragmentation back to reactants. In the case of cyclopentenone and isobutene,
both the relative rates of addition and the fraction proceeding on to product have been
determined. The preferred regioisomer results from a larger fraction of cyclization for
the intermediate with radical character at the
-carbon. 129
O
O
CH 3
. 4%
CH 3
.
O. C(CH ) 2 - 3% yield
=C(CH ) 3 2
CH 2 3 2 7 –1 –1
. 4.8 x 10 M s
7
–1 –1
2.6 x 10 M s
O . O
C(CH ) 21%
3 2
.
CH 3
CH 3
20% yield
Significantly, both cis and trans ring junctures are formed. 130 Cyclohexenone and
cyclopentene, for example, give four adducts with about a 2:1 cis:trans ratio. 131
O O H H
O O HH
O H H HH
hv
+
HH H H
H H H H 25%
68% < 1% 7%
127
P. de Mayo, Acc. Chem. Res., 4, 41 (1971); D. I. Schuster, G. Lem, and N. A. Kaprinidis, Chem. Rev.,
93, 3 (1993).
128 D. I. Schuster, D. A. Dunn, G. E. Heibel, P. B. Brown, J. M. Rao, J. Woning, and R. Bonnneau, J. Am.
Chem. Soc., 113, 6245 (1991).
129
D. Andrew, D. J. Hastings, D. L. Oldroyd, A. Rudolph, A. C. Weedon, D. F. Wong, and B. Zhang,
Pure Appl. Chem., 64, 1327 (1992); D. Andrew, D. J. Hastings, and A. C. Weedon, J. Am. Chem. Soc.,
116, 10870 (1994); D. Andrew and A. C. Weedon, J. Am. Chem. Soc., 117, 5647 (1995).
130 E. J. Corey, J. D. Bass, R. Le Mahieu, and R. B. Mitra, J. Am. Chem. Soc., 86, 5570 (1964).
131
D. I. Schuster, N. Kaprinidis, D. J. Wink, and J. C. Dewan, J. Org. Chem., 56, 561 (1991).