Page 1137 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 1137
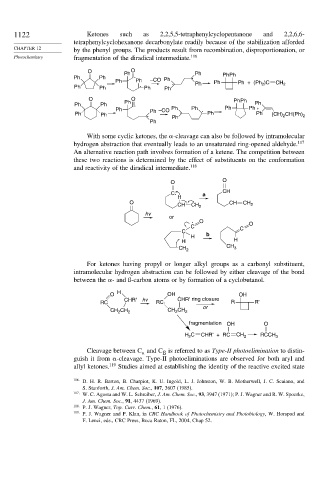
1122 Ketones such as 2,2,5,5-tetraphenylcyclopentanone and 2,2,6,6-
tetraphenylcyclohexanone decarbonylate readily because of the stabilization afforded
CHAPTER 12 by the phenyl groups. The products result from recombination, disproportionation, or
Photochemistry fragmentation of the diradical intermediate. 116
O Ph O Ph
Ph Ph . . PhPh
Ph Ph –CO Ph . Ph Ph Ph + (Ph )C CH
Ph Ph . Ph Ph 2 2
O O PhPh
Ph Ph Ph . Ph
Ph –CO Ph Ph . Ph Ph +
Ph Ph . Ph Ph . Ph Ph (CH) 2 CH(Ph) 2
Ph
With some cyclic ketones, the -cleavage can also be followed by intramolecular
hydrogen abstraction that eventually leads to an unsaturated ring-opened aldehyde. 117
An alternative reaction path involves formation of a ketene. The competition between
these two reactions is determined by the effect of substituents on the conformation
and reactivity of the diradical intermediate. 118
O O
CH
C. a
H
O . CH CH
CH CH 2 2
hv
or
. O O
C
C C
H b
H H
. CH
CH 2 3
For ketones having propyl or longer alkyl groups as a carbonyl substituent,
intramolecular hydrogen abstraction can be followed by either cleavage of the bond
between the - and ß-carbon atoms or by formation of a cyclobutanol.
H
O OH . OH
CHR' hv CHR' ring closure
RC RC. R R'
or
CH CH 2 CH 2 CH 2
2
fragmentation OH O
H C CHR' + RC CH 2 RCCH 3
2
Cleavage between C and C is referred to as Type-II photoelimination to distin-
ß
guish it from -cleavage. Type-II photoeliminations are observed for both aryl and
allyl ketones. 119 Studies aimed at establishing the identity of the reactive excited state
116 D. H. R. Barton, B. Charpiot, K. U. Ingold, L. J. Johnston, W. B. Motherwell, J. C. Scaiano, and
S. Stanforth, J. Am. Chem. Soc., 107, 3607 (1985).
117
W. C. Agosta and W. L. Schreiber, J. Am. Chem. Soc., 93, 3947 (1971); P. J. Wagner and R. W. Spoerke,
J. Am. Chem. Soc., 91, 4437 (1969).
118 P. J. Wagner, Top. Curr. Chem., 61, 1 (1976).
119
P. J. Wagner and P. Klan, in CRC Handbook of Photochemistry and Photobiology, W. Horspod and
F. Lenci, eds., CRC Press, Boca Raton, FL, 2004, Chap 52.