Page 1132 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 1132
∗
∗
∗
excited states corresponding to n → , → , and n → have been described, and 1117
y z
there are several Rydberg states as well. The atomic populations and bond orders were
calculated using the AIM method. SECTION 12.3
Photochemistry of
Carbonyl Compounds
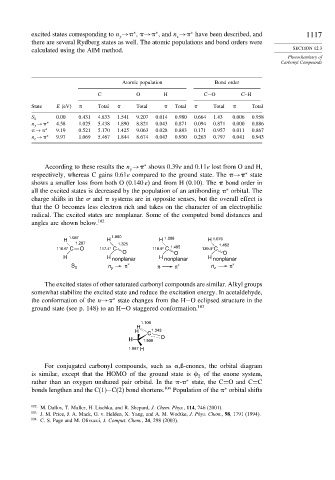
Atomic population Bond order
C O H C−O C–H
State E (eV) Total Total Total Total Total
0.00 0.431 4.833 1.541 9.207 0.014 0.980 0.664 1.43 0.006 0.958
S 0
n y → ∗ 4.58 1.025 5.438 1.890 8.821 0.043 0.871 0.094 0.871 0.000 0.886
→ ∗ 9.19 0.521 5.170 1.425 9.063 0.028 0.883 0.171 0.957 0.011 0.867
n z → ∗ 9.97 1.069 5.467 1.844 8.674 0.043 0.930 0.263 0.797 0.041 0.943
∗
According to these results the n → shows 0.39e and 0.11e lost from O and H,
y
respectively, whereas C gains 0.61e compared to the ground state. The → state
∗
shows a smaller loss from both O (0.140 e) and from H (0.10). The bond order in
all the excited states is decreased by the population of an antibonding orbital. The
∗
charge shifts in the and systems are in opposite senses, but the overall effect is
that the O becomes less electron rich and takes on the character of an electrophilic
radical. The excited states are nonplanar. Some of the computed bond distances and
angles are shown below. 102
1.087 1.090 1.088
H H H H 1.076
1.207 1.325 1.462
116.6° C O 117.4° C 118.9° C 1.495 139.4°C
O O O
H H nonplanar H nonplanar H nonplanar
n π ∗ π π ∗ n π ∗
S 0 y z
The excited states of other saturated carbonyl compounds are similar. Alkyl groups
somewhat stabilize the excited state and reduce the excitation energy. In acetaldehyde,
the conformation of the n→ state changes from the H−O eclipsed structure in the
∗
ground state (see p. 148) to an H−O staggered conformation. 103
1.106
H
H C 1.343
H 1.500 O
1.087 H
For conjugated carbonyl compounds, such as ,ß-enones, the orbital diagram
is similar, except that the HOMO of the ground state is of the enone system,
2
∗
rather than an oxygen unshared pair orbital. In the - state, the C=O and C=C
∗
bonds lengthen and the C(1)−C(2) bond shortens. 104 Population of the orbital shifts
102
M. Dallos, T. Muller, H. Lischka, and R. Shepard, J. Chem. Phys., 114, 746 (2001).
103 J. M. Price, J. A. Mack, G. v. Helden, X. Yang, and A. M. Wodtke, J. Phys. Chem., 98, 1791 (1994).
104
C. S. Page and M. Olivucci, J. Comput. Chem., 24, 298 (2003).