Page 1130 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 1130
The resistance of the 3-unsubstituted system to the di- -methane rearrangement 1115
probably occurs at the stage of the vinyl rearrangement. 95 If the central carbon is
unsubstituted this step results in the formation of a primary radical and would be SECTION 12.2
energetically unfavorable. Photochemistry of
Alkenes, Dienes, and
Kinetic studies of 3a, 3b, and 3c have provided experimental evidence that a Polyenes
cyclopropyl diradical is an intermediate. 96 The product composition reveals a temper-
ature dependence that implicates an intermediate. These compounds undergo both
cis-trans isomerization and di- -methane rearrangement on direct irradiation and
mainly cis-trans isomerization when photosensitized (triplet) by benzophenone. The
quantum yield of the di- -methane rearrangement increases dramatically with substi-
tution at the allylic position.
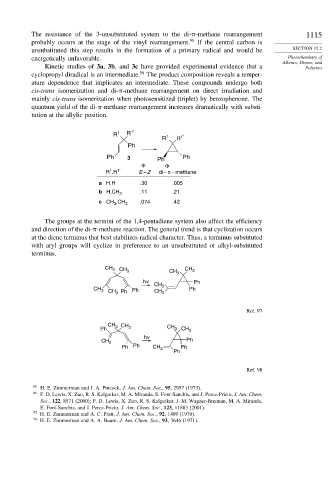
R 1 R 1′ 1 1′
R R
Ph
Ph 3 Ph Ph
Φ Φ
1
R ,R 1′ E – Z di – π – methane
a H,H .30 .005
b H,CH 3 .11 .21
c CH ,CH 3 .074 .42
3
The groups at the termini of the 1,4-pentadiene system also affect the efficiency
and direction of the di- -methane reaction. The general trend is that cyclization occurs
at the diene terminus that best stabilizes radical character. Thus, a terminus substituted
with aryl groups will cyclize in preference to an unsubstituted or alkyl-substituted
terminus.
CH 3 CH CH
3 CH 3 3
hv Ph
CH 3
CH 3 CH 3 Ph Ph CH 3 Ph
Ref. 97
CH 3 CH
Ph 3 CH 3 CH 3
hv
CH 2 Ph
Ph Ph CH 2 Ph
Ph
Ref. 98
95
H. E. Zimmerman and J. A. Pincock, J. Am. Chem. Soc., 95, 2957 (1973).
96
F. D. Lewis, X. Zuo, R. S. Kalgutkar, M. A. Miranda, E. Font-Sanchis, and J. Perez-Prieto, J. Am. Chem.
Soc., 122, 8571 (2000); F. D. Lewis, X. Zuo, R. S. Kalgutkar, J. M. Wagner-Brennan, M. A. Miranda,
E. Font-Sanchis, and J. Perez-Prieto, J. Am. Chem. Soc., 123, 11883 (2001).
97 H. E. Zimmerman and A. C. Pratt, J. Am. Chem. Soc., 92, 1409 (1970).
98
H. E. Zimmerman and A. A. Baum, J. Am. Chem. Soc., 93, 3646 (1971).