Page 1127 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 1127
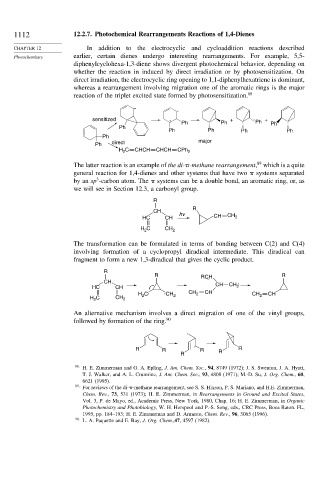
1112 12.2.7. Photochemical Rearrangements Reactions of 1,4-Dienes
CHAPTER 12 In addition to the electrocyclic and cycloaddition reactions described
Photochemistry earlier, certain dienes undergo interesting rearrangements. For example, 5,5-
diphenylcyclohexa-1,3-diene shows divergent photochemical behavior, depending on
whether the reaction in induced by direct irradiation or by photosensitization. On
direct irradiation, the electrocyclic ring opening to 1,1-diphenylhexatriene is dominant,
whereas a rearrangement involving migration one of the aromatic rings is the major
reaction of the triplet excited state formed by photosensitization. 88
.
.
sensitized . + +
Ph Ph Ph Ph
Ph Ph Ph
. Ph Ph
Ph
Ph direct major
H C CHCH CHCH CPh 2
2
89
The latter reaction is an example of the di- -methane rearrangement, which is a quite
general reaction for 1,4-dienes and other systems that have two systems separated
3
by an sp -carbon atom. The systems can be a double bond, an aromatic ring, or, as
we will see in Section 12.3, a carbonyl group.
R
R
CH
hv
HC CH CH CH 2
C CH
H 2 2
The transformation can be formulated in terms of bonding between C(2) and C(4)
involving formation of a cyclopropyl diradical intermediate. This diradical can
fragment to form a new 1,3-diradical that gives the cyclic product.
R
R R
RCH .
CH .
HC CH CH CH 2
H C . . CH CH 2 CH CH CH
H C CH 2 2 2 2
2
An alternative mechanism involves a direct migration of one of the vinyl groups,
followed by formation of the ring. 90
.
.
R R R R
R R
88
H. E. Zimmerman and G. A. Epling, J. Am. Chem. Soc., 94, 8749 (1972); J. S. Swenton, J. A. Hyatt,
T. J. Walker, and A. L. Crumrine, J. Am. Chem. Soc., 93, 4808 (1971); M.-D. Su, J. Org. Chem., 60,
6621 (1995).
89 For reviews of the di- -methane rearrangement, see S. S. Hixson, P. S. Mariano, and H.E. Zimmerman,
Chem. Rev., 73, 531 (1973); H. E. Zimmerman, in Rearrangements in Ground and Excited States,
Vol. 3, P. de Mayo, ed., Academic Press, New York, 1980, Chap. 16; H. E. Zimmerman, in Organic
Photochemistry and Photobiology, W. H. Horspool and P.-S. Song, eds., CRC Press, Boca Raton. FL,
1995, pp. 184–193; H. E. Zimmerman and D. Armesto, Chem. Rev., 96, 3065 (1996).
90
L. A. Paquette and E. Bay, J. Org. Chem.,47, 4597 (1982).