Page 1123 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 1123
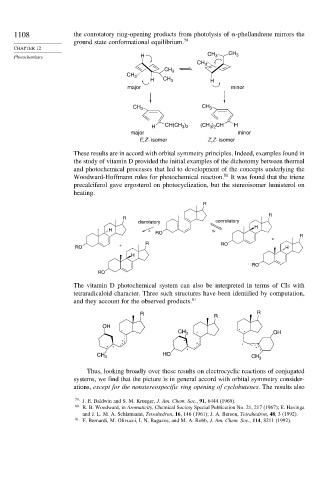
1108 the conrotatory ring-opening products from photolysis of -phellandrene mirrors the
ground state conformational equilibrium. 79
CHAPTER 12
Photochemistry H CH 3 CH 3
CH 3
CH 3
CH 3
H CH 3 H
major minor
CH 3 CH 3
H CH(CH ) (CH 3 ) 2 CH H
3 2
major minor
E,Z- isomer Z,Z- isomer
These results are in accord with orbital symmetry principles. Indeed, examples found in
the study of vitamin D provided the initial examples of the dichotomy between thermal
and photochemical processes that led to development of the concepts underlying the
Woodward-Hoffmann rules for photochemical reaction. 80 It was found that the triene
precalciferol gave ergosterol on photocyclization, but the stereoisomer lumisterol on
heating.
R
R
R
disrotatory conrotatory
H
H Δ hν
RO R
+
R RO
+
RO H
H
RO
RO
The vitamin D photochemical system can also be interpreted in terms of CIs with
tetraradicaloid character. Three such structures have been identified by computation,
and they account for the observed products. 81
R R
R
OH
. CH 3 . . . OH
. . .
. . . .
.
CH 3 HO CH 3
Thus, looking broadly over these results on electrocyclic reactions of conjugated
systems, we find that the picture is in general accord with orbital symmetry consider-
ations, except for the nonstereospecific ring opening of cyclobutenes. The results also
79
J. E. Baldwin and S. M. Krueger, J. Am. Chem. Soc., 91, 6444 (1969).
80 R. B. Woodward, in Aromaticity, Chemical Society Special Publication No. 21, 217 (1967); E. Havinga
and J. L. M. A. Schlatmann, Tetrahedron, 16, 146 (1961); J. A. Berson, Tetrahedron, 48, 3 (1992).
81
F. Bernardi, M. Olivucci, I. N. Ragazos, and M. A. Robb, J. Am. Chem. Soc., 114, 8211 (1992).