Page 1117 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 1117
1102 S 2 S 1
CHAPTER 12 10
Photochemistry
44 18
18
hv
18
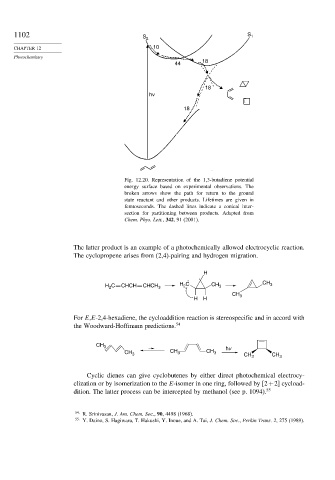
Fig. 12.20. Representation of the 1,3-butadiene potential
energy surface based on experimental observations. The
broken arrows show the path for return to the ground
state reactant and other products. Lifetimes are given in
femtoseconds. The dashed lines indicate a conical inter-
section for partitioning between products. Adapted from
Chem. Phys. Lett., 342, 91 (2001).
The latter product is an example of a photochemically allowed electrocyclic reaction.
The cyclopropene arises from (2,4)-pairing and hydrogen migration.
H
. .
C CHCH CHCH H C CH CH 3
H 2 3 2 3
CH
H H 3
For E,E-2,4-hexadiene, the cycloaddition reaction is stereospecific and in accord with
the Woodward-Hoffmann predictions. 54
CH 3 hv
CH 3 CH 3 CH 3 CH 3 CH 3
Cyclic dienes can give cyclobutenes by either direct photochemical electrocy-
clization or by isomerization to the E-isomer in one ring, followed by [2+2] cycload-
dition. The latter process can be intercepted by methanol (see p. 1094). 55
54 R. Srinivasan, J. Am. Chem. Soc., 90, 4498 (1968).
55
Y. Daino, S. Hagiwara, T. Hakushi, Y. Inoue, and A. Tai, J. Chem. Soc., Perkin Trans. 2, 275 (1989).